Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter15: Radical Reactions
Section: Chapter Questions
Problem 12E
Related questions
Question
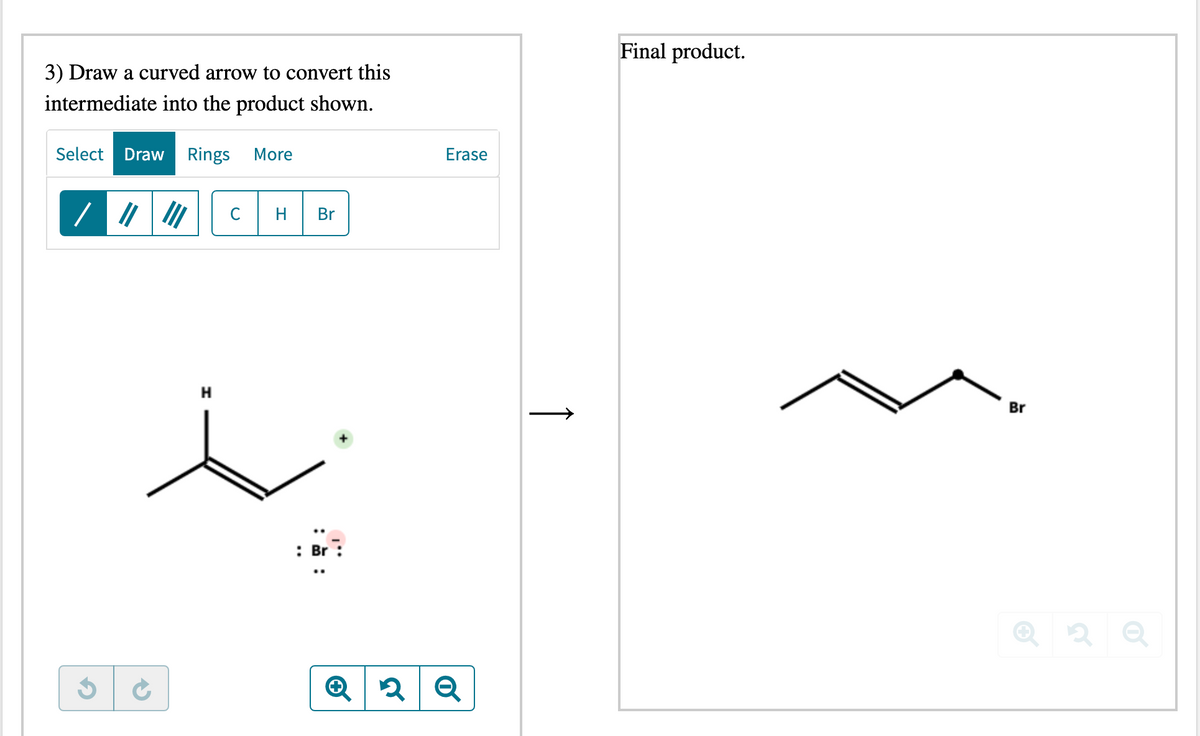
Transcribed Image Text:Final product.
3) Draw a curved arrow to convert this
intermediate into the product shown.
Select
Draw
Rings
More
Erase
II
C
H
Br
H
Br
: Br
↑
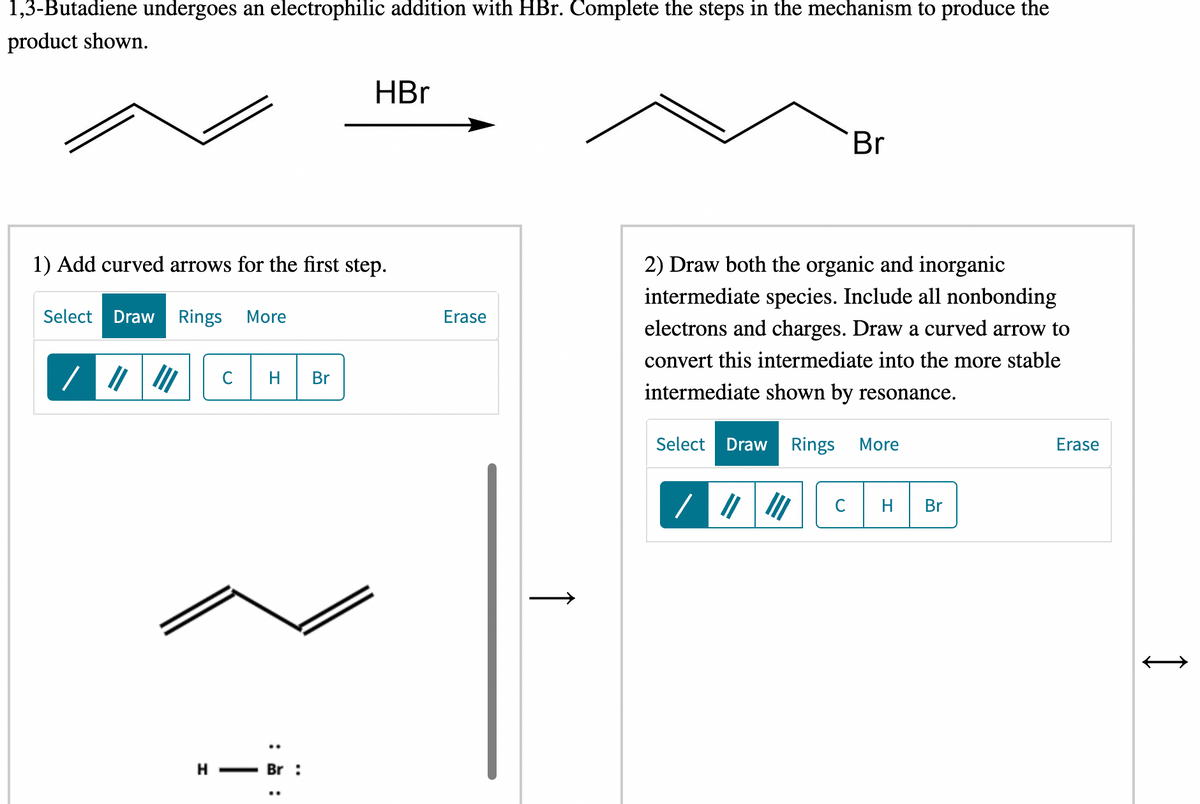
Transcribed Image Text:1,3-Butadiene undergoes an electrophilic addition with HBr. Complete the steps in the mechanism to produce the
product shown.
HBr
Br
2) Draw both the organic and inorganic
intermediate species. Include all nonbonding
1) Add curved arrows for the first step.
Select
Draw
Rings
More
Erase
electrons and charges. Draw a curved arrow to
convert this intermediate into the more stable
C
H
Br
intermediate shown by resonance.
Select Draw Rings
More
Erase
H
Br
..
Br :
↑
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning