13. potassium oxide + calcium bromide → calcium oxide + potassium bromide K₂O + CaBr₂ → CaO + KBr 14. methane, CH4, burns in oxygen to produce carbon monoxide and water CH4 + O₂ → CO₂ + H₂O 15. ethane undergoes complete combustion C2H4 + O2 → CO₂ + H₂O chved
13. potassium oxide + calcium bromide → calcium oxide + potassium bromide K₂O + CaBr₂ → CaO + KBr 14. methane, CH4, burns in oxygen to produce carbon monoxide and water CH4 + O₂ → CO₂ + H₂O 15. ethane undergoes complete combustion C2H4 + O2 → CO₂ + H₂O chved
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 97E
Related questions
Question
100%
please balance equations 13-15
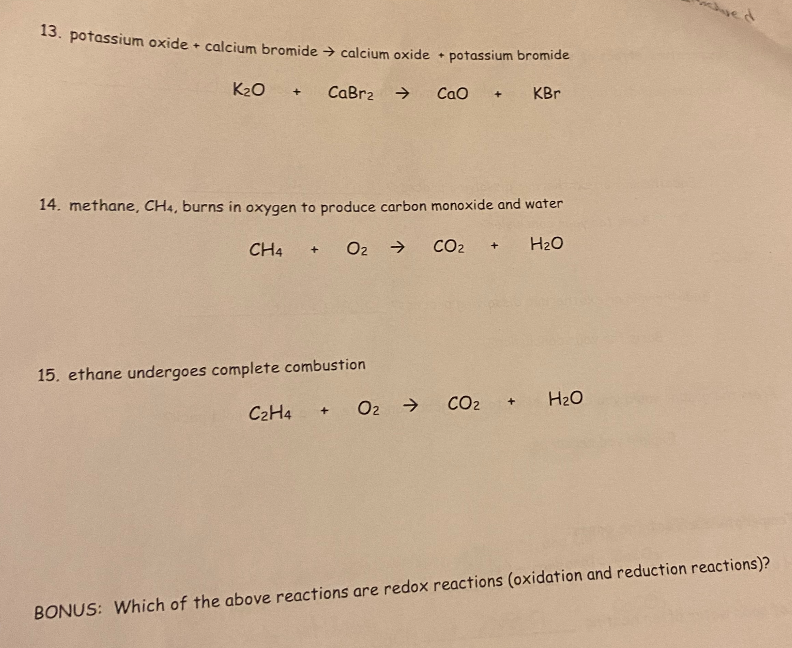
Transcribed Image Text:13. potassium oxide + calcium bromide → calcium oxide + potassium bromide
K₂O + CaBr2 →
CaO + KBr
14. methane, CH4, burns in oxygen to produce carbon monoxide and water
O₂ →
H₂O
CH4
+
15. ethane undergoes complete combustion
C2H4
+ O₂ →
CO₂
+
CO₂ +
H₂O
BONUS: Which of the above reactions are redox reactions (oxidation and reduction reactions)?
Expert Solution
Step 1
In order to balance any chemical equation, it is very important to make equal number of atoms in reactant and product sides.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning