13.2) Draw the line bond structure for the general form of an amino acid using "R" to represent the side chain. Draw the N- and C- terminus groups using the forms that are predominant at physiological pH (pH=7.4). Include lone pairs in your drawing. quaternary ammonium group Quaternary ammonium groups that are attached to the a-carbons of amino acids have pKą values of about 9.5, therefore, at pH = 7.4, the acid form (quaternary ammonium group) is predominant. ANSWER: H H H-N-C H R :0: || C carboxylate group The pK values of amino acid carboxyl groups are between 2 and 5 (depending on which amino acid), therefore, at pH = 7.4, the base form (carboxylate ion) is predominant. R-group (called "side-chain")
13.2) Draw the line bond structure for the general form of an amino acid using "R" to represent the side chain. Draw the N- and C- terminus groups using the forms that are predominant at physiological pH (pH=7.4). Include lone pairs in your drawing. quaternary ammonium group Quaternary ammonium groups that are attached to the a-carbons of amino acids have pKą values of about 9.5, therefore, at pH = 7.4, the acid form (quaternary ammonium group) is predominant. ANSWER: H H H-N-C H R :0: || C carboxylate group The pK values of amino acid carboxyl groups are between 2 and 5 (depending on which amino acid), therefore, at pH = 7.4, the base form (carboxylate ion) is predominant. R-group (called "side-chain")
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter21: Organic And Biological Molecules
Section: Chapter Questions
Problem 10RQ
Related questions
Question
100%
I don't get it at all. I struggled with my homework. Can you help me about the Henderson-Hasselbalch relation, summarized in the table below, it is possible to predict the predominant form of these groups at a particular pH? Draw the line bond structure for the general form of an amino acid using "R" to represent the side chain. Draw the N- and C-terminus groups using the forms that are predominant at physiological pH (pH = 7.4. Include lone pairs in your drawing.
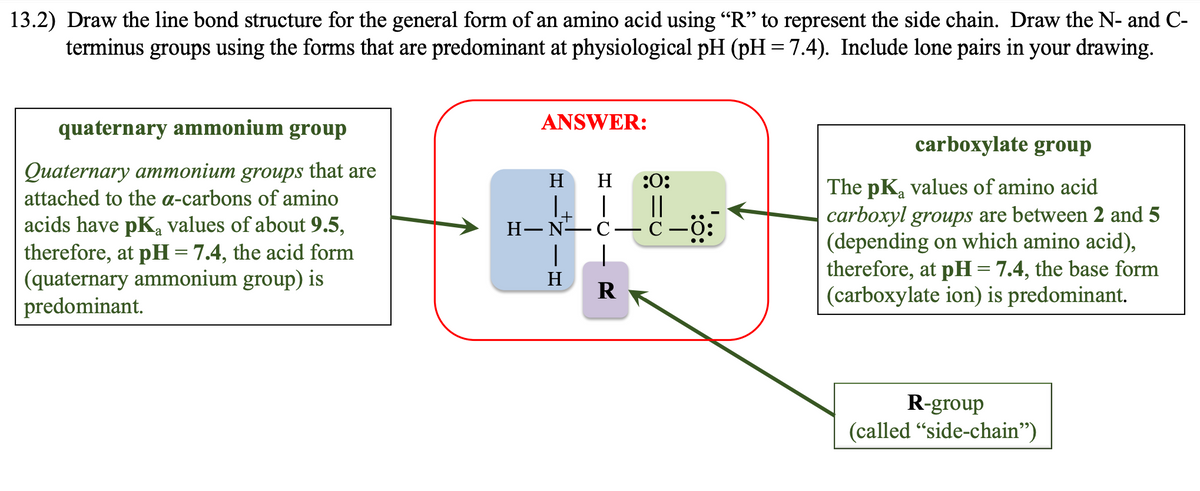
Transcribed Image Text:13.2) Draw the line bond structure for the general form of an amino acid using “R” to represent the side chain. Draw the N- and C-
terminus groups using the forms that are predominant at physiological pH (pH = 7.4). Include lone pairs in your drawing.
quaternary ammonium group
Quaternary ammonium groups that are
attached to the a-carbons of amino
acids have pK₂ values of about 9.5,
therefore, at pH = 7.4, the acid form
(quaternary ammonium group) is
predominant.
ANSWER:
H :0:
||
H-N—C—C—0:
H
H
R
carboxylate group
The pKa values of amino acid
carboxyl groups are between 2 and 5
(depending on which amino acid),
therefore, at pH = 7.4, the base form
(carboxylate ion) is predominant.
R-group
(called "side-chain")
![Draw the line bond structure for the general form of an amino acid using "R" to represent the side chain. Draw the N- and C-
terminus groups using the forms that are predominant at physiological pH (pH = 7.4). Include lone pairs in your drawing.
HINT:
The structure of the predominant form of an amino acid will depend on the pH because amino acids
involve the carboxyl group/carboxylate group conjugate pair and the quaternary ammonium
group/amine group conjugate pair. Using the implications of the the Henderson-Hasselbalch
relation, summarized in the table below, it is possible to predict the predominant form of these
groups at a particular pH.
Solution
Condition
pH <pka
pH > pKa
pH = pka
Relative Amounts of
Acid and Base Forms
[HA] > [A-]
[A-] > [HA]
[HA] = [A-]
The pK₂ values of amino acid carboxyl groups are between 2 and 5 (depending on which amino
acid), therefore, at pH = 7.4, the base form (carboxylate ion) is predominant.
Quaternary ammonium groups that are attached to the a-carbons of amino acids have pk₁ values of
about 9.5, therefore, at pH = 7.4, the acid form (quaternary ammonium group) is predominant.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F3442c249-7c73-43ca-b651-caace2a5bb24%2F7b70351f-1657-451c-9025-3cfa72cb8d1e%2Fpmikoqg_processed.png&w=3840&q=75)
Transcribed Image Text:Draw the line bond structure for the general form of an amino acid using "R" to represent the side chain. Draw the N- and C-
terminus groups using the forms that are predominant at physiological pH (pH = 7.4). Include lone pairs in your drawing.
HINT:
The structure of the predominant form of an amino acid will depend on the pH because amino acids
involve the carboxyl group/carboxylate group conjugate pair and the quaternary ammonium
group/amine group conjugate pair. Using the implications of the the Henderson-Hasselbalch
relation, summarized in the table below, it is possible to predict the predominant form of these
groups at a particular pH.
Solution
Condition
pH <pka
pH > pKa
pH = pka
Relative Amounts of
Acid and Base Forms
[HA] > [A-]
[A-] > [HA]
[HA] = [A-]
The pK₂ values of amino acid carboxyl groups are between 2 and 5 (depending on which amino
acid), therefore, at pH = 7.4, the base form (carboxylate ion) is predominant.
Quaternary ammonium groups that are attached to the a-carbons of amino acids have pk₁ values of
about 9.5, therefore, at pH = 7.4, the acid form (quaternary ammonium group) is predominant.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning