1301 decays by emission of beta particles to form stable 130xe. A 3.00 g lodine sample containing some I-130 was recorded as having 5652 disintegrations per min. k = 0.00094 min 1, How many radioactive l-130 atoms are present in the sample? Answer:
1301 decays by emission of beta particles to form stable 130xe. A 3.00 g lodine sample containing some I-130 was recorded as having 5652 disintegrations per min. k = 0.00094 min 1, How many radioactive l-130 atoms are present in the sample? Answer:
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter12: Kinetics
Section: Chapter Questions
Problem 46E: Fluorine-18 is a radioactive isotope that decays by positron emission to form oxygen-18 with a...
Related questions
Question
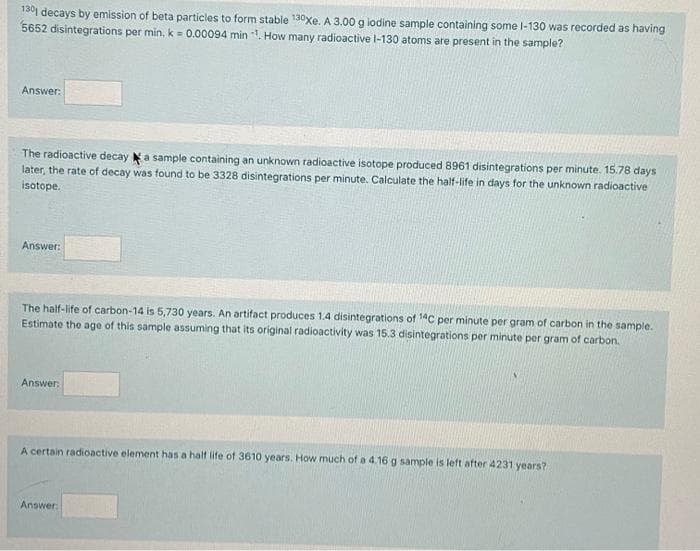
Transcribed Image Text:130 decays by emission of beta particles to form stable 130xe. A 3.00 g lodine sample containing some I-130 was recorded as having
5652 disintegrations per min, k = 0.00094 min . How many radioactive l-130 atoms are present in the sample?
Answer:
The radioactive decay a sample containing an unknown radioactive isotope produced 8961 disintegrations per minute. 15.78 days
later, the rate of decay was found to be 3328 disintegrations per minute. Calculate the half-life in days for the unknown radioactive
isotope.
Answer:
The half-life of carbon-14 is 5,730 years. An artifact produces 1.4 disintegrations of 14C per minute per gram of carbon in the sample.
Estimate the age of this sample assuming that its original radioactivity was 15.3 disintegrations per minute per gram of carbon.
Answer:
A certain radioactive element has a half life of 3610 years. How much of a 4.16 g sample is left after 4231 years?
Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co