Carbon-14 (C-14) is a radioactive isotope with a of 5,730 years. It decays to stable through beta decay. Which process half-life nitrogen-14 (N-14) is a current use of C-14? producing energy in nuclear power plants determining the age of buried organic materials C reducing the size of certain tumors generating radiation for medical imaging Save/Exit https://www.oncourseconnect.com/assessment/1692307/94132404-a95a-1326-6d99-db8581b65beb#
Carbon-14 (C-14) is a radioactive isotope with a of 5,730 years. It decays to stable through beta decay. Which process half-life nitrogen-14 (N-14) is a current use of C-14? producing energy in nuclear power plants determining the age of buried organic materials C reducing the size of certain tumors generating radiation for medical imaging Save/Exit https://www.oncourseconnect.com/assessment/1692307/94132404-a95a-1326-6d99-db8581b65beb#
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter19: Nuclear Chemistry
Section: Chapter Questions
Problem 50AP
Related questions
Question
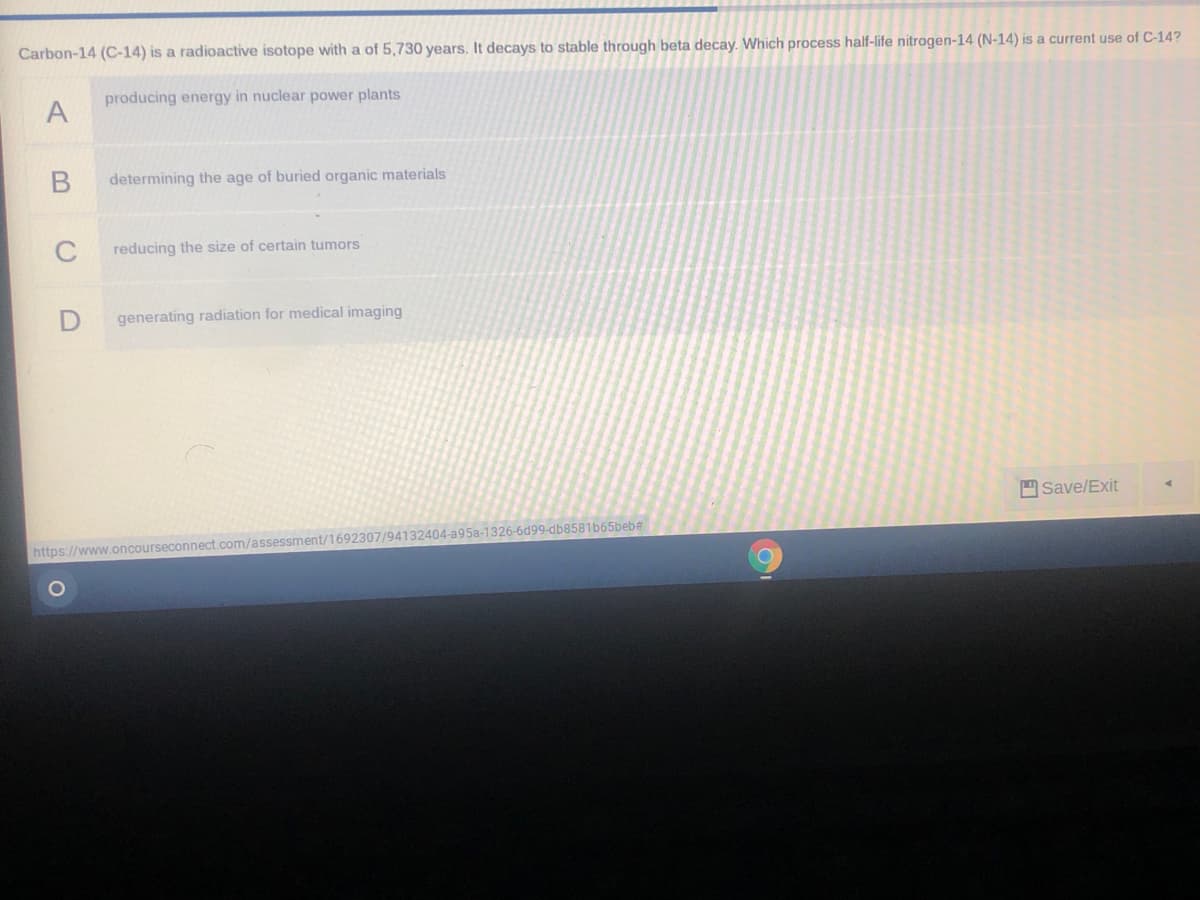
Transcribed Image Text:Carbon-14 (C-14) is a radioactive isotope with a of 5,730 years, It decays to stable through beta decay. Which process half-life nitrogen-14 (N-14) is a current use of C-14?
producing energy in nuclear power plants
determining the age of buried organic materials
C
reducing the size of certain tumors
generating radiation for medical imaging
Save/Exit
https://www.oncourseconnect.com/assessment/1692307/94132404-a95a-1326-6d99-db8581b65beb#
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning