135 ban 21. Which one of the compounds below could NOT be the limiting reactant in the following reaction? Thood K4Fe(CN)6+ H₂SO4+H₂O → K2SO4 + FeSO4 + (NH4)2SO4 + CO a. K4Fe(CN)6 e. none of these can be the limiting reactant c. H₂O d. FeSO4 b. H₂SO4 rool hoitoon to a W Sastow bas at 22. How many moles of oxygen gas can be made if you start with 2 moles of potassium chlorate not (KCIO3)? norteadmoos RXN: 2 KCIO3 → 3 0₂ 2 KCl + 74.6 g/mol 122.6g/mol 32g/mol et end not stargacy of a. 1 mol b. 2 mol c. 3 mol d. 4 mol 14 23. Balancing of equations satisfies which law? a. Law of Conservation of Energy anon lo yram wor bad insisvos signie a ol al b. Law of Elements c. Law of Reactions 2 d. Law of Conservation of Mass 5 liens C 0 [
135 ban 21. Which one of the compounds below could NOT be the limiting reactant in the following reaction? Thood K4Fe(CN)6+ H₂SO4+H₂O → K2SO4 + FeSO4 + (NH4)2SO4 + CO a. K4Fe(CN)6 e. none of these can be the limiting reactant c. H₂O d. FeSO4 b. H₂SO4 rool hoitoon to a W Sastow bas at 22. How many moles of oxygen gas can be made if you start with 2 moles of potassium chlorate not (KCIO3)? norteadmoos RXN: 2 KCIO3 → 3 0₂ 2 KCl + 74.6 g/mol 122.6g/mol 32g/mol et end not stargacy of a. 1 mol b. 2 mol c. 3 mol d. 4 mol 14 23. Balancing of equations satisfies which law? a. Law of Conservation of Energy anon lo yram wor bad insisvos signie a ol al b. Law of Elements c. Law of Reactions 2 d. Law of Conservation of Mass 5 liens C 0 [
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 148AE: Commercial brass, an alloy of Zn and Cu, reacts with hydrochloric acid os follows:...
Related questions
Question
need a answer to all of these please and thank you
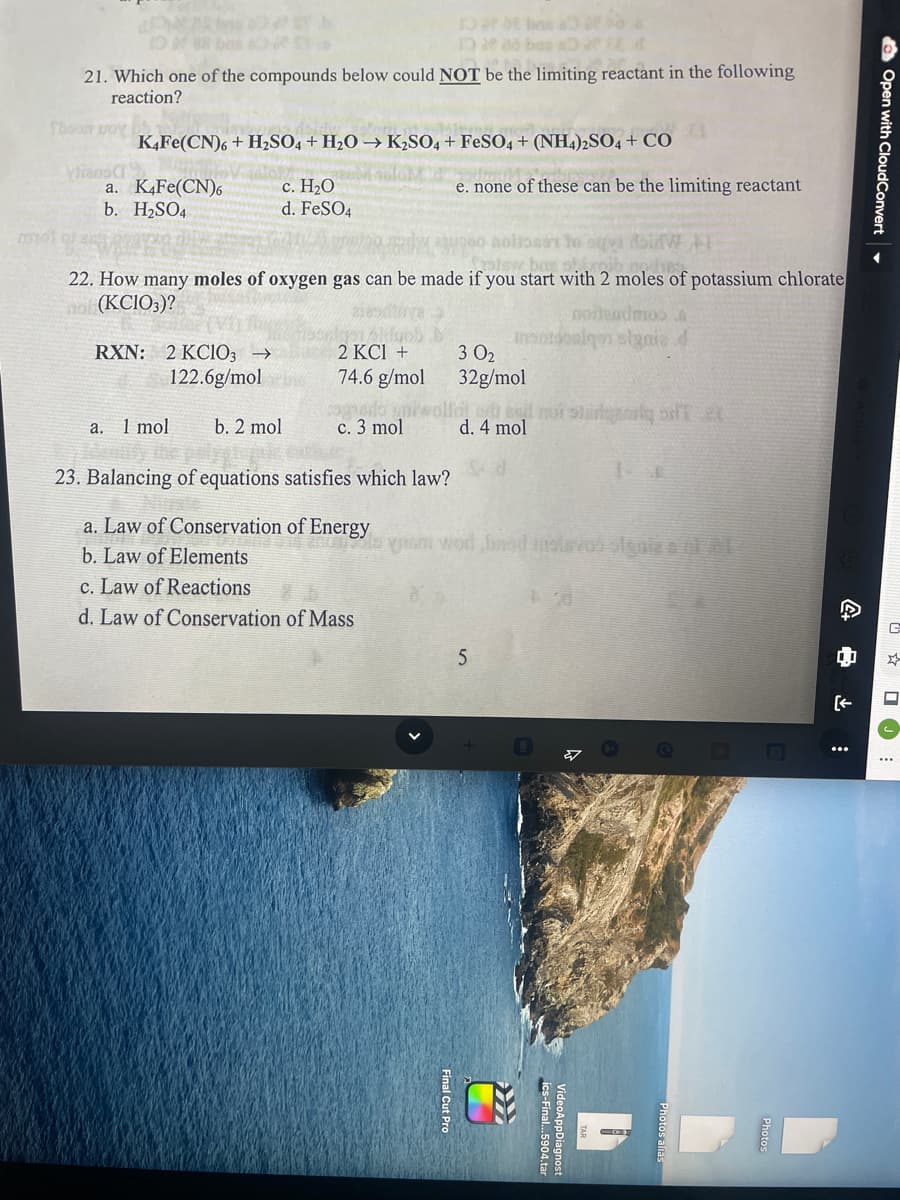
Transcribed Image Text:TODE be a fo
10 88 bas
21. Which one of the compounds below could NOT be the limiting reactant in the following
reaction?
Thood boy
K4Fe(CN)6+ H₂SO4 + H₂O → K₂SO4 + FeSO4 + (NH4)2SO4 + CO
dians C
a. K4Fe(CN)6
e. none of these can be the limiting reactant
c. H₂O
d. FeSO4
b. H₂SO4
mnot
molteon
22. How many moles of oxygen gas can be made if you start with 2 moles of potassium chlorate
not (KCIO3)?
HONORA
norteadmos s
eigen signie d
RXN: 2 KC1O3 →
3 0₂
2 KCl +
74.6 g/mol
122.6g/mol
32g/mol
ogrodo
fol or end not start of
d. 4 mol
a. 1 mol
b. 2 mol
c. 3 mol
23. Balancing of equations satisfies which law?
a. Law of Conservation of Energy
yam wod band insisvoo signies of al
b. Law of Elements
c. Law of Reactions
d. Law of Conservation of Mass
5
Final Cut Pro
ics-Final 5904
VideoAppDiagn
4
个
Open with CloudConvert
C
☆
...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
