14. A CHEM 106 student made a 55.0 % by mass sodium sulfate aqueous solution at 50 °C (Assume the density of water is 1.00 g/mL). a. The solubility of sodium sulfate aqueous solution at 50 °C is (use the provided plot): b. Is the solution made by the student undersaturated, saturated or supersaturated? provide all the necessary calculations to prove your response. Solubility (g solute in 100 g H₂O) 100 90 80 70 60 50 40 30 20 10 0 NaNO, Pb(NO3)2 NaCl CaCl₂ 0 10 20 30 KNO, Na₂SO4 KCI KCIO, 40 50 60 Temperature (°C) 70 K₂Cr₂O; 80 90 100
14. A CHEM 106 student made a 55.0 % by mass sodium sulfate aqueous solution at 50 °C (Assume the density of water is 1.00 g/mL). a. The solubility of sodium sulfate aqueous solution at 50 °C is (use the provided plot): b. Is the solution made by the student undersaturated, saturated or supersaturated? provide all the necessary calculations to prove your response. Solubility (g solute in 100 g H₂O) 100 90 80 70 60 50 40 30 20 10 0 NaNO, Pb(NO3)2 NaCl CaCl₂ 0 10 20 30 KNO, Na₂SO4 KCI KCIO, 40 50 60 Temperature (°C) 70 K₂Cr₂O; 80 90 100
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 9P
Related questions
Question
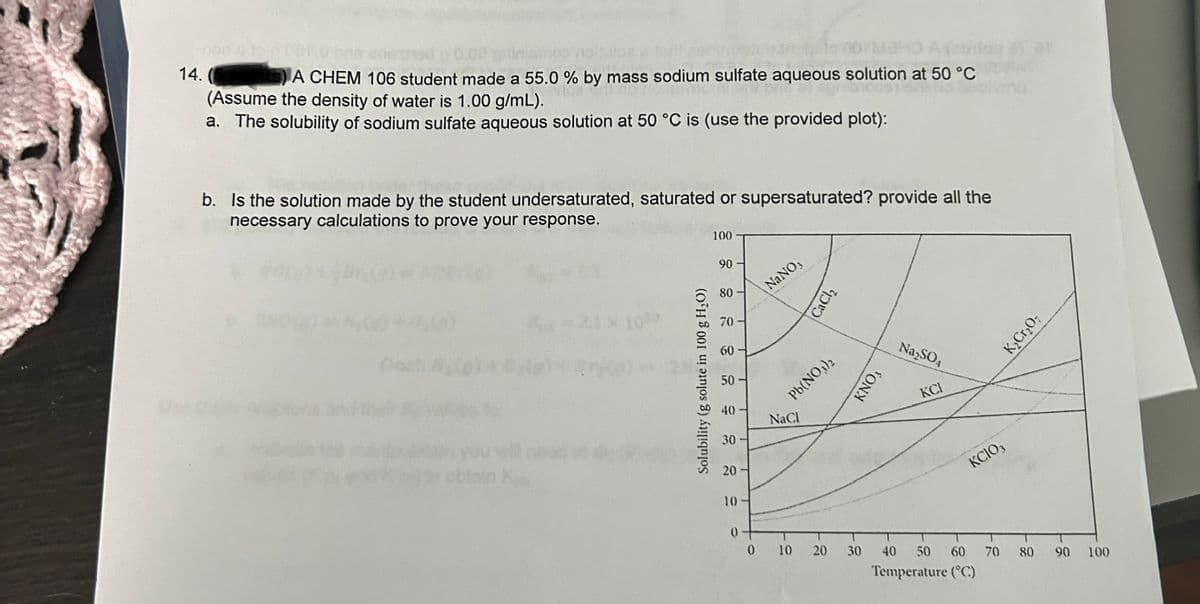
Transcribed Image Text:14.
stoppor
0.09 priniainoa noituloa a fort
ts) A CHEM 106 student made a 55.0 % by mass sodium sulfate aqueous solution at 50 °C
(Assume the density of water is 1.00 g/mL).
a. The solubility of sodium sulfate aqueous solution at 50 °C is (use the provided plot):
b. Is the solution made by the student undersaturated, saturated or supersaturated? provide all the
necessary calculations to prove your response.
103
Solubility (g solute in 100 g H₂O)
100
90
80
70
60
40
30
20
10
0
0
NaNO3
Pb(NO3)2
NaCl
CaCl₂
10
20
30
ΚΝΟ,
Na₂SO4
KCI
KCIO3
K₂Cr₂O7
40 50 60 70 80 90
Temperature (°C)
100

Transcribed Image Text:15. (5
Calculate the vapor pressure (in torr) at 25 °C of a solution containing 49.8 mL ethylene glycol
(HOCH₂CH₂OH; a non-volatile compound; density=1.11g/mL) and 285.2 g water. The vapor pressure of
pure water at 25 °C is 23.8 torr.
loa olbos inevica.
HA HA terit ensem (HA) noitulos to volertine almortoxe nA
ami rouol of bloo alestat bas
dulce e nerv
Expert Solution
Step 1
Solutions-
The given graph shows the solubility of all substances. we have to find the solubility of Na2SO4.
We also find the vapor pressure of solution containing ethylene glycol.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning