140 Belt of Stability 130 120 110 = Stable Isotope 100 Number of Neutrons 50 50 70 60 60 70 80 60 90 90 40 40 30 20 20 10 10 0 0 10 20 20 30 60 70 10 40 50 Number of Protons 80 90
140 Belt of Stability 130 120 110 = Stable Isotope 100 Number of Neutrons 50 50 70 60 60 70 80 60 90 90 40 40 30 20 20 10 10 0 0 10 20 20 30 60 70 10 40 50 Number of Protons 80 90
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter19: Radioactivity And Nuclear Energy
Section: Chapter Questions
Problem 11QAP
Related questions
Question
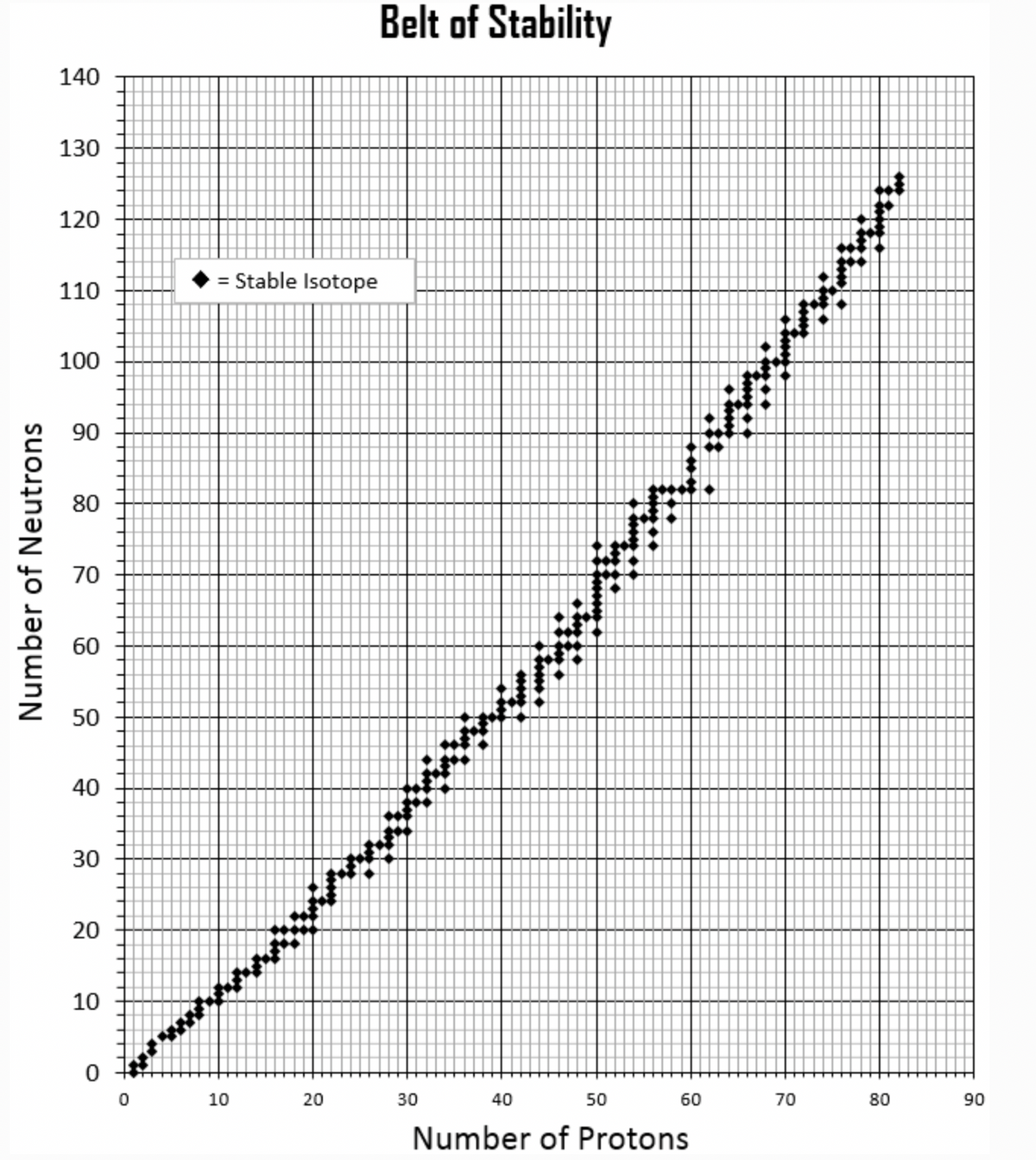
Based on their neutron to proton ratio, what type of nuclear decay would you expect for each of the following isotopes. Are the isotopes stable?
Mg-30
Cd-113
Transcribed Image Text:140
Belt of Stability
130
120
110
= Stable Isotope
100
Number of Neutrons
50
50
70
60
60
70
80
60
90
90
40
40
30
20
20
10
10
0
0
10
20
20
30
60
70
10
40
50
Number of Protons
80
90
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning