A SodaStream "sparkling water" home carbonation system pressurizes a 1L water bottle to a pressure of 4 bar (400 kPa) in order to dissolve CO2 gas into the liquid to produce carbonated water also known as "Fizzy-Water". Small bubbles are injected to promote mass transfer such that we can assume the liquid-gas system is at equilibrium with water at a typical refrigerator temperature of 2 °C = 275 K. Assuming that both CO2 and water are ideal gases and CO2 is minimally soluble in water such that Henry's Law is an appropriate model for behavior, what mass of CO2 is dissolved in a 1L water bottle and what would be the equivalent volume, in liters, of that mass if released at room temperature of T = 298 K and pressure of 4 bar (during pressurization) and at 1 bar (when drinking the water)? Assume solvent MH20 18.015 kg/kmol, Psat@2c = 2 kPa == solute Mco₂ = 44.01 kg/kmol, Rco₂ = 0.1889 kPa m³/kgK, Hco₂ = 510 bar, Hint: The mass fraction and mole fraction of a mixture are related to each other by (where the subscript m is for "mixture"). = Mi mi = Wi mm Ni Mi NmMm Yi Mm
A SodaStream "sparkling water" home carbonation system pressurizes a 1L water bottle to a pressure of 4 bar (400 kPa) in order to dissolve CO2 gas into the liquid to produce carbonated water also known as "Fizzy-Water". Small bubbles are injected to promote mass transfer such that we can assume the liquid-gas system is at equilibrium with water at a typical refrigerator temperature of 2 °C = 275 K. Assuming that both CO2 and water are ideal gases and CO2 is minimally soluble in water such that Henry's Law is an appropriate model for behavior, what mass of CO2 is dissolved in a 1L water bottle and what would be the equivalent volume, in liters, of that mass if released at room temperature of T = 298 K and pressure of 4 bar (during pressurization) and at 1 bar (when drinking the water)? Assume solvent MH20 18.015 kg/kmol, Psat@2c = 2 kPa == solute Mco₂ = 44.01 kg/kmol, Rco₂ = 0.1889 kPa m³/kgK, Hco₂ = 510 bar, Hint: The mass fraction and mole fraction of a mixture are related to each other by (where the subscript m is for "mixture"). = Mi mi = Wi mm Ni Mi NmMm Yi Mm
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Plz explain, no handwritten answer
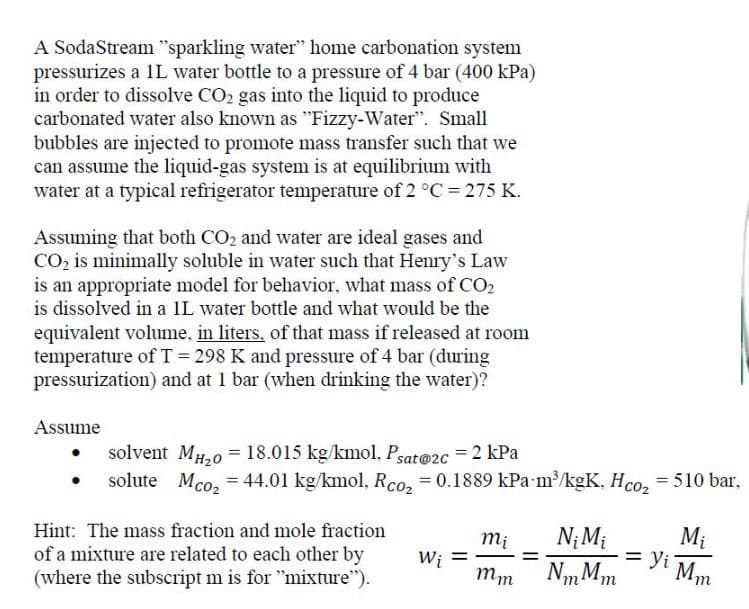
Transcribed Image Text:A SodaStream "sparkling water" home carbonation system
pressurizes a 1L water bottle to a pressure of 4 bar (400 kPa)
in order to dissolve CO2 gas into the liquid to produce
carbonated water also known as "Fizzy-Water". Small
bubbles are injected to promote mass transfer such that we
can assume the liquid-gas system is at equilibrium with
water at a typical refrigerator temperature of 2 °C = 275 K.
Assuming that both CO2 and water are ideal gases and
CO2 is minimally soluble in water such that Henry's Law
is an appropriate model for behavior, what mass of CO2
is dissolved in a 1L water bottle and what would be the
equivalent volume, in liters, of that mass if released at room
temperature of T = 298 K and pressure of 4 bar (during
pressurization) and at 1 bar (when drinking the water)?
Assume
solvent MH20 18.015 kg/kmol, Psat@2c = 2 kPa
==
solute Mco₂ = 44.01 kg/kmol, Rco₂ = 0.1889 kPa m³/kgK, Hco₂ = 510 bar,
Hint: The mass fraction and mole fraction
of a mixture are related to each other by
(where the subscript m is for "mixture").
=
Mi
mi
=
Wi
mm
Ni Mi
NmMm
Yi Mm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY