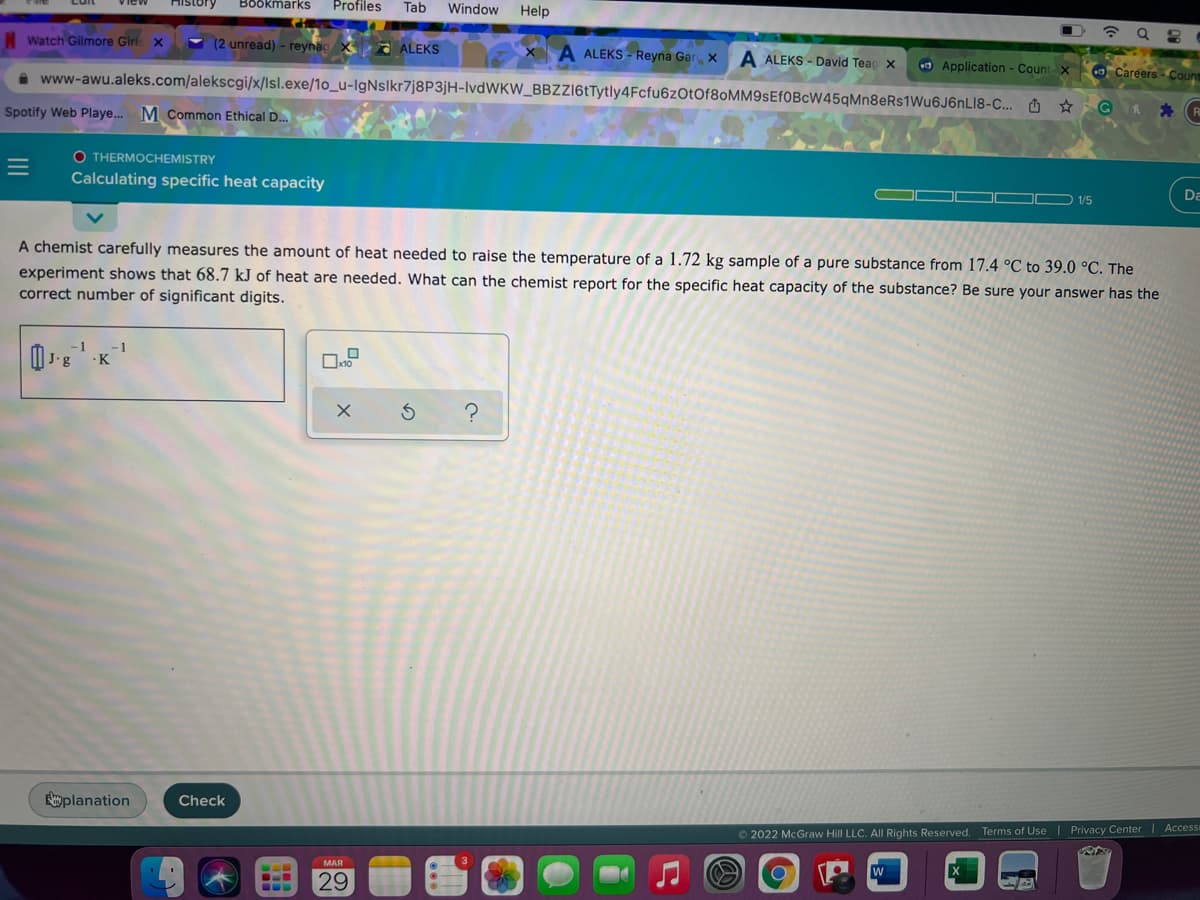
1/5 A chemist carefully measures the amount of heat needed to raise the temperature of a 1.72 kg sample of a pure substance from 17.4 °C to 39.0 °C. The experiment shows that 68.7 kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance? Be sure your answer has the correct number of significant digits.
1/5 A chemist carefully measures the amount of heat needed to raise the temperature of a 1.72 kg sample of a pure substance from 17.4 °C to 39.0 °C. The experiment shows that 68.7 kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance? Be sure your answer has the correct number of significant digits.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter34: Particle Size Determination
Section: Chapter Questions
Problem 34.12QAP
Related questions
Question
Transcribed Image Text:Bookmarks
Profiles
Tab
Window
Help
N Watch Gilmore Girl x
Y (2 unread) - reynac X ALEKS
A ALEKS - Reyna Gar x
A ALEKS - David Teac x
O Application - Count x
O Careers - Count
i www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IvdWKW_BBZZ16tTytly4Fcfu6zOtOf80MM9sEf0BcW45qMn8eRs1Wu6J6nLI8-C... O * GA * E
Spotify Web Playe... M Common Ethical D..
O THERMOCHEMISTRY
Calculating specific heat capacity
Da
1/5
A chemist carefully measures the amount of heat needed to raise the temperature of a 1.72 kg sample of a pure substance from 17.4 °C to 39.0 °C. The
experiment shows that 68.7 kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance? Be sure your answer has the
correct number of significant digits.
1
·K
pplanation
Check
Access
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use| Privacy Center
MAR
W
29
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning