15. Identiiy the substance being oxidized in the following equation: 2 Al + Fe203 Al203 + 2 Fe (b) iron(III) ion (c) oxide ion (a) aluminum (d) iron metal
15. Identiiy the substance being oxidized in the following equation: 2 Al + Fe203 Al203 + 2 Fe (b) iron(III) ion (c) oxide ion (a) aluminum (d) iron metal
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 9QAP: Write balanced net ionic equations for the following reactions in acid solution. (a) Liquid...
Related questions
Question
#15
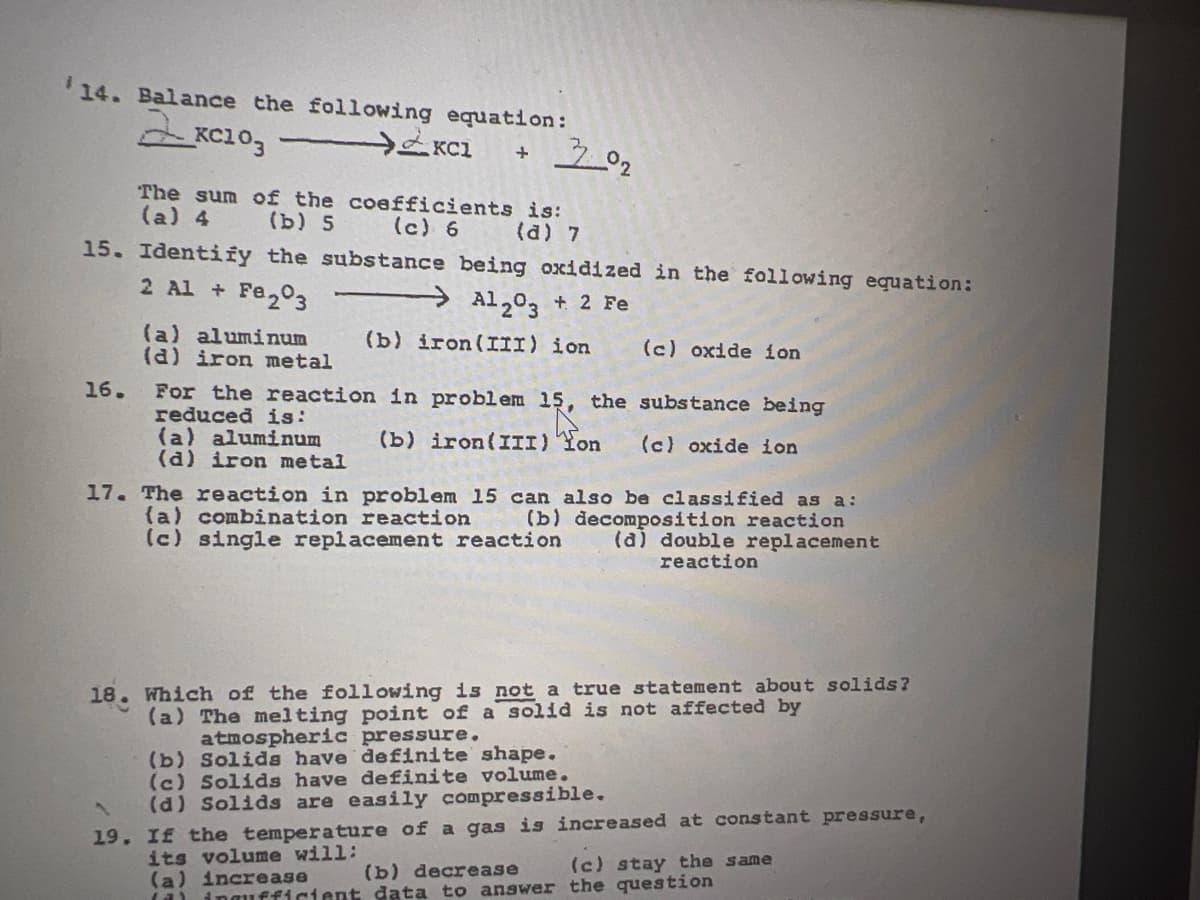
Transcribed Image Text:14. Balance the following equation:
The sum of the coefficients is:
(a) 4
(b) 5
(c) 6
(a) 7
15. Identify the substance being oxidized in the following equation:
2 Al + Fe203
Al203 + 2 Fe
(a) aluminum
(d) iron metal
(b) iron(III) ion
(c) oxide ion
16.
For the reaction in problem 15, the substance being
reduced is:
(a) aluminum
(d) iron metal
(b) iron(III) Yon
(c) oxide ion
17. The reaction in problem 15 can also be classified as a:
(a) combination reaction
(c) single replacement reaction
(b) decomposition reaction
(a) double replacement
reaction
18. Which of the following is not a true statement about solids?
(a) The melting point of a solid is not affected by
atmospheric pressure.
(b) Solida have definite shape.
(c) Solids have definite volume.
(d) Solids are easily compressible.
19. If the temperature of a gas is increased at constant pressure,
its volume will:
(a) increase
(a) ingufficient data to answer the question
(b) decrease
(c) stay the same
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
