15.3) In the previous problem, you drew the products for the phosphorylation of ADP reaction (shown below). Does that reacti require energy or release energy when it occurs? ANSWER: This reaction requires energy in order to occur. phosphoryl group O-P OH inorganic phosphate (P₁) the OCH₂ H H H H OH OH adenosine diphosphate (ADP) NH₂ + H+ O O =+++R O -OCH₂ H H O H OH OH adenosine triphosphate (ATP) H NH₂ + H₂O
15.3) In the previous problem, you drew the products for the phosphorylation of ADP reaction (shown below). Does that reacti require energy or release energy when it occurs? ANSWER: This reaction requires energy in order to occur. phosphoryl group O-P OH inorganic phosphate (P₁) the OCH₂ H H H H OH OH adenosine diphosphate (ADP) NH₂ + H+ O O =+++R O -OCH₂ H H O H OH OH adenosine triphosphate (ATP) H NH₂ + H₂O
Biology: The Dynamic Science (MindTap Course List)
4th Edition
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Chapter6: Energy, Enzymes, And Biological Reactions
Section: Chapter Questions
Problem 16TYK
Related questions
Question
100%
I don't understand it. Can u help me? Can u help me to explain this to me, please
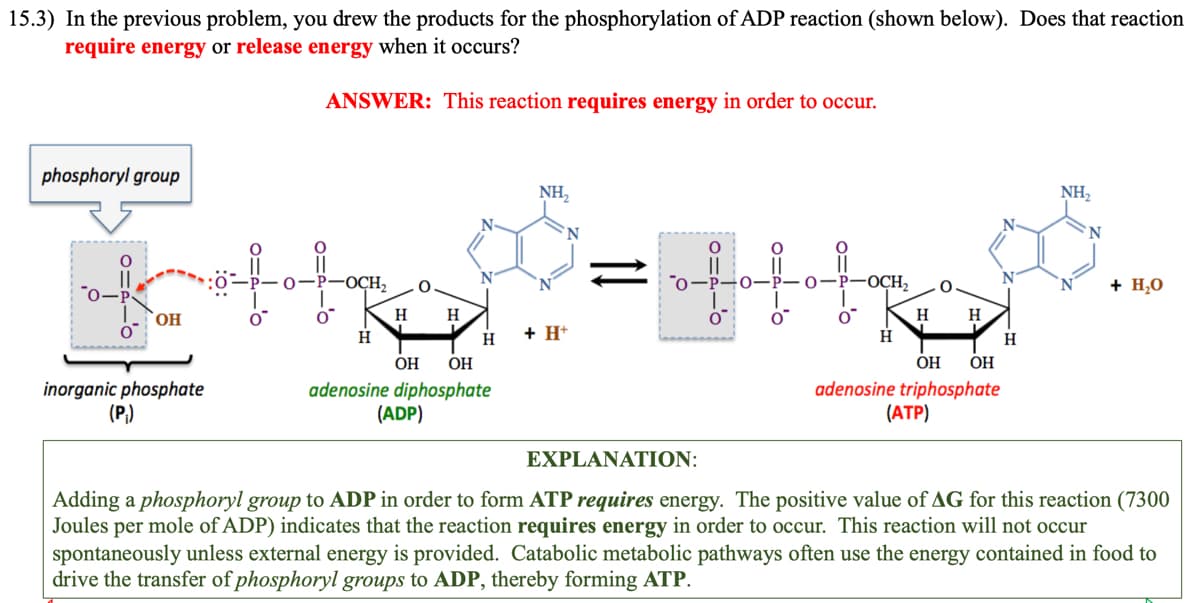
Transcribed Image Text:15.3) In the previous problem, you drew the products for the phosphorylation of ADP reaction (shown below). Does that reaction
require energy or release energy when it occurs?
ANSWER: This reaction requires energy in order to occur.
phosphoryl group
OH
inorganic phosphate
(P₁)
0=2-0
O
0=4-'0
P-OCH₂
H
H
O
H
N
H
OH OH
adenosine diphosphate
(ADP)
NH₂
+ H+
fol
O-P-O
O
-OCH₂
H
H
O
H
N
OH OH
adenosine triphosphate
(ATP)
H
NH₂
+ H₂O
EXPLANATION:
Adding a phosphoryl group to ADP in order to form ATP requires energy. The positive value of AG for this reaction (7300
Joules per mole of ADP) indicates that the reaction requires energy in order to occur. This reaction will not occur
spontaneously unless external energy is provided. Catabolic metabolic pathways often use the energy contained in food to
drive the transfer of phosphoryl groups to ADP, thereby forming ATP.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax