(16%) Problem 3: The diagram presented represents a thermodynamic process experienced by 28.1 mmol (millimoles) of a monatomic ideal gas. The volume axis is divided into equal increments vo=817 cm³ while the pressure axis is divided into equal increments Po 0.851 atm. 4 po - 3 po 2 po 1 po - + + +- + + -1- 30 400 V 0 100 200 How much work, in joules, does the gas perform on its environment during the thermodynamic process represented in the diagram? What is the change, in joules, of the internal energy of the gas during the process represented in the diagram? How much heat, in joules, is absorbed by the gas during the process represented in the diagram?
(16%) Problem 3: The diagram presented represents a thermodynamic process experienced by 28.1 mmol (millimoles) of a monatomic ideal gas. The volume axis is divided into equal increments vo=817 cm³ while the pressure axis is divided into equal increments Po 0.851 atm. 4 po - 3 po 2 po 1 po - + + +- + + -1- 30 400 V 0 100 200 How much work, in joules, does the gas perform on its environment during the thermodynamic process represented in the diagram? What is the change, in joules, of the internal energy of the gas during the process represented in the diagram? How much heat, in joules, is absorbed by the gas during the process represented in the diagram?
Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 76P: (a) An ideal gas expands adiabatically from a volume of 2.0103 m3 to 2.5103 m3. If the initial...
Related questions
Question
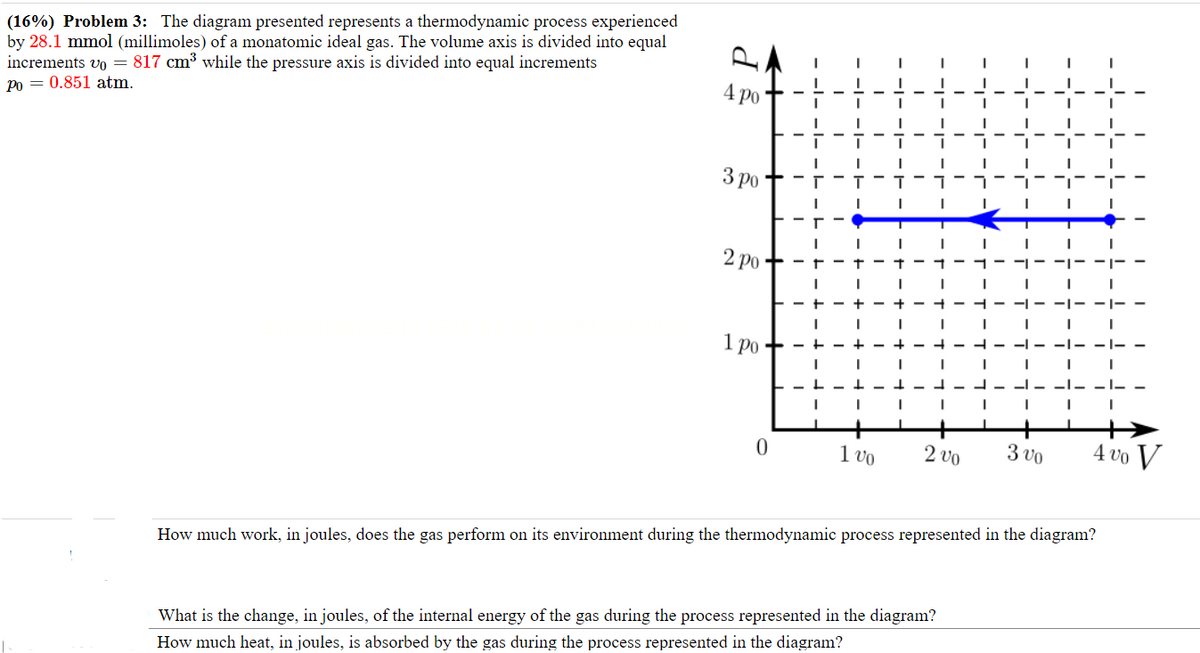
Transcribed Image Text:(16%) Problem 3: The diagram presented represents a thermodynamic process experienced
by 28.1 mmol (millimoles) of a monatomic ideal gas. The volume axis is divided into equal
increments vo=817 cm³ while the pressure axis is divided into equal increments
Po 0.851 atm.
4 po
-
3 po
2 po
1 po
-
+
+
+-
+
+
-1-
30
400 V
0
100
200
How much work, in joules, does the gas perform on its environment during the thermodynamic process represented in the diagram?
What is the change, in joules, of the internal energy of the gas during the process represented in the diagram?
How much heat, in joules, is absorbed by the gas during the process represented in the diagram?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning