18. What patterns do you see in all of the compound models? Elements having 1,2 or 3 valence electrons (electropositive elements) transfer their valence electrons to electronegative elements like N,0,F, Ci, forming an ionic bond.
18. What patterns do you see in all of the compound models? Elements having 1,2 or 3 valence electrons (electropositive elements) transfer their valence electrons to electronegative elements like N,0,F, Ci, forming an ionic bond.
Chapter82: Physical Constants Of Liquids: The Boiling Point And Density
Section: Chapter Questions
Problem 5P
Related questions
Question
Question 18 is wrong can you please fix it for me
Transcribed Image Text:Almustafa, Mays
y Co of SAs 1-ocx
2001 at74 p
912)
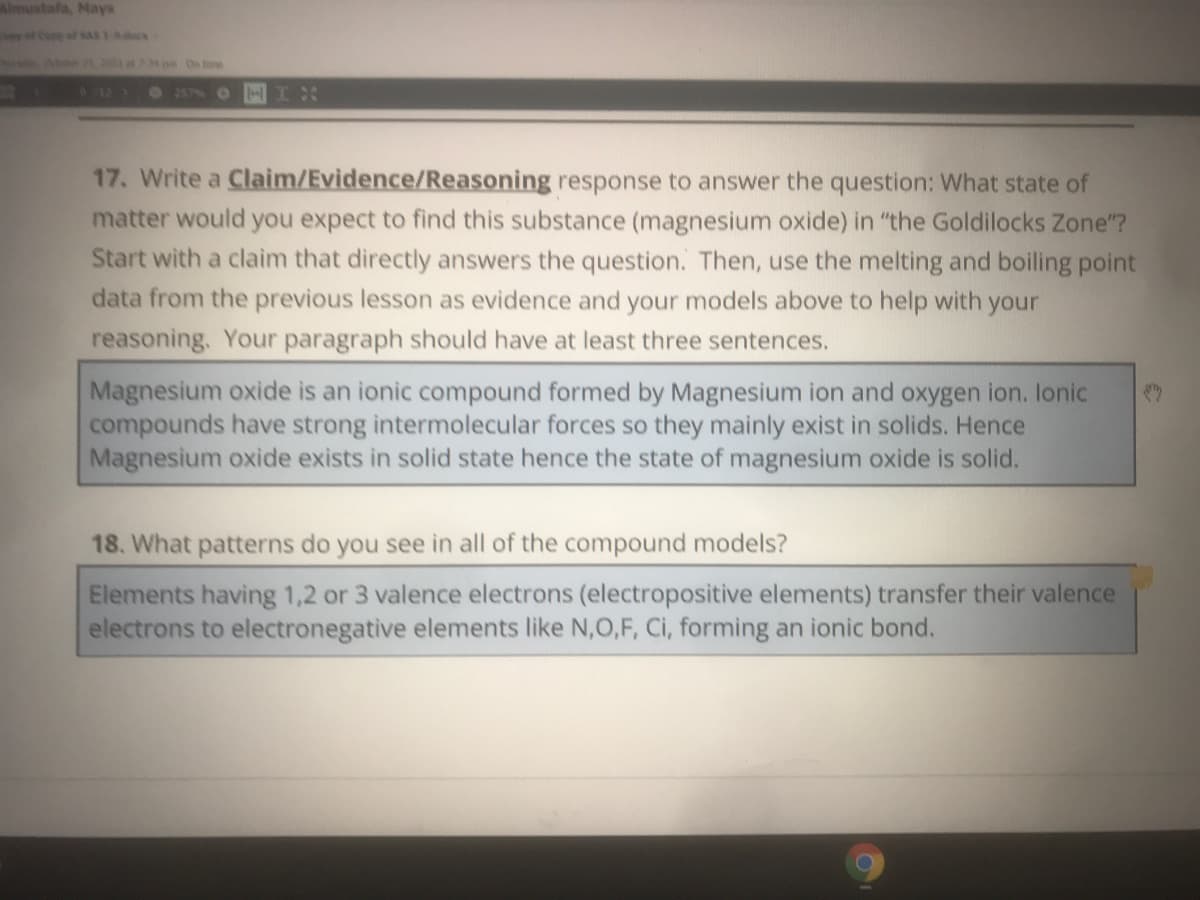
17. Write a Claim/Evidence/Reasoning response to answer the question: What state of
matter would you expect to find this substance (magnesium oxide) in "the Goldilocks Zone"?
Start with a claim that directly answers the question. Then, use the melting and boiling point
data from the previous lesson as evidence and your models above to help with your
reasoning. Your paragraph should have at least three sentences.
Magnesium oxide is an ionic compound formed by Magnesium ion and oxygen ion. lonic
compounds have strong intermolecular forces so they mainly exist in solids. Hence
Magnesium oxide exists in solid state hence the state of magnesium oxide is solid.
18. What patterns do you see in all of the compound models?
Elements having 1,2 or 3 valence electrons (electropositive elements) transfer their valence
electrons to electronegative elements like N,0,F, Ci, forming an ionic bond.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning