18.9 milliliters of a 1.687 M KOH solution are required to titrate 30.1 milliliters of a H3PO4 solution. What is the molarity of the H3PO4 solution? 3KOH + H3PO4 --> K3PO4+ 3H2O Please put answer in box with 3 digits past decimal in regular non scientific notation.
18.9 milliliters of a 1.687 M KOH solution are required to titrate 30.1 milliliters of a H3PO4 solution. What is the molarity of the H3PO4 solution? 3KOH + H3PO4 --> K3PO4+ 3H2O Please put answer in box with 3 digits past decimal in regular non scientific notation.
Chapter9: Acids, Bases, And Salts
Section: Chapter Questions
Problem 9.101E
Related questions
Question
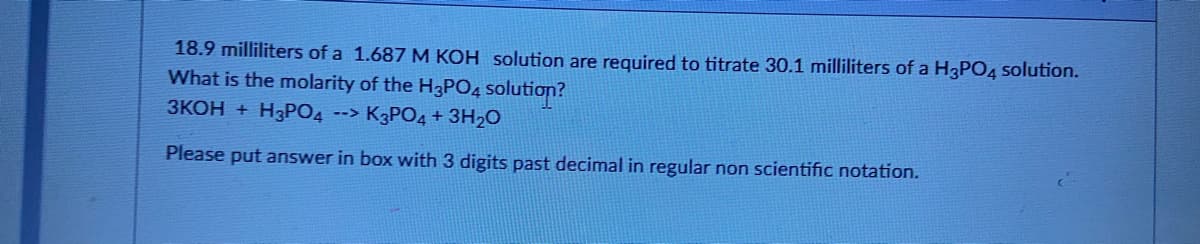
Transcribed Image Text:18.9 milliliters of a 1.687 M KOH solution are required to titrate 30.1 milliliters of a H3PO4 solution.
What is the molarity of the H3PO4 solution?
ЗКОН + HзРОД
--> K3PO4 + 3H2O
Please put answer in box with 3 digits past decimal in regular non scientific notation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning