19.) Predict the effect of the changes of the following exothermic reaction at equilibrium by applying Le Châtelier's Principle. Does the reaction shift right (towards product), left (towards reactants), or no effect? CO (g) + Cl₂ (g) a.) adding Cl2 to the reaction mixture b.) removing COCl2 to the reaction mixture c.) removing CO from the reaction mixture d.) decreasing the reaction temperature. e.) increasing the reaction volume. COCI₂ (g)
19.) Predict the effect of the changes of the following exothermic reaction at equilibrium by applying Le Châtelier's Principle. Does the reaction shift right (towards product), left (towards reactants), or no effect? CO (g) + Cl₂ (g) a.) adding Cl2 to the reaction mixture b.) removing COCl2 to the reaction mixture c.) removing CO from the reaction mixture d.) decreasing the reaction temperature. e.) increasing the reaction volume. COCI₂ (g)
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter14: Chemical Equilibirum
Section: Chapter Questions
Problem 14.23QP: For the reaction 2HI(g)H2(g)+I2(g) carried out at some fixed temperature, the equilibrium constant...
Related questions
Question
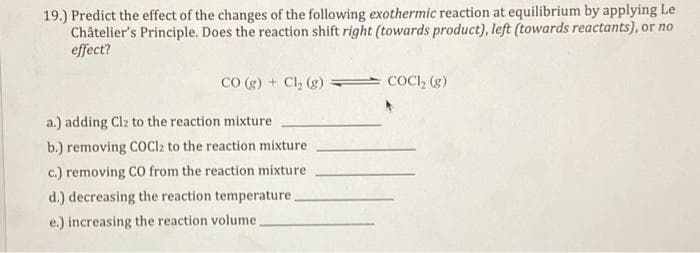
Transcribed Image Text:19.) Predict the effect of the changes of the following exothermic reaction at equilibrium by applying Le
Châtelier's Principle. Does the reaction shift right (towards product), left (towards reactants), or no
effect?
CO (g) + Cl₂ (g)
a.) adding Cl2 to the reaction mixture
b.) removing COCl2 to the reaction mixture
c.) removing CO from the reaction mixture
d.) decreasing the reaction temperature.
e.) increasing the reaction volume.
COCI₂ (g)
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning