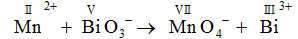Chemistry 9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Steven S. Zumdahl
1 Chemical Foundations 2 Atoms, Molecules, And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8 Bonding: General Concepts 9 Covalent Bonding: Orbitals 10 Liquids And Solids 11 Properties Of Solutions 12 Chemical Kinetics 13 Chemical Equilibrium 14 Acids And Bases 15 Acid-base Equilibria 16 Solubility And Complex Ion Equilibria 17 Spontaneity, Entropy, And Free Energy 18 Electrochemistry 19 The Nucleus: A Chemist's View 20 The Representative Elements 21 Transition Metals And Coordination Chemistry 22 Organic And Biological Molecules Chapter18: Electrochemistry
Chapter Questions Section: Chapter Questions
Problem 1RQ: What is a half-reaction? Why must the number of electrons lost in the oxidation half reaction equal... Problem 2RQ: Galvanic cells harness spontaneous oxidationreduction reactions to produce work by producing a... Problem 3RQ: Table 17-1 lists common half-reactions along with the standard reduction potential associated with... Problem 4RQ: Consider the equation G = -nF. What are the four terms in this equation? Why does a minus sign... Problem 5RQ: The Nernst equation allows determination of the cell potential for a galvanic cell at nonstandard... Problem 6RQ: What are concentration cells? What is in a concentration cell? What is the driving force for a... Problem 7RQ Problem 8RQ Problem 9RQ: What characterizes an electrolytic cell? What is an ampere? When the current applied to an... Problem 1ALQ: Sketch a galvanic cell, and explain how it works. Look at Figs. 17-1 and 17-2. Explain what is... Problem 2ALQ: In making a specific galvanic cell, explain how one decides on the electrodes and the solutions to... Problem 3ALQ Problem 4ALQ Problem 5ALQ: Sketch a cell that forms iron metal from iron(II) while changing chromium metal to chromium(III).... Problem 6ALQ: Which of the following is the best reducing agent: F2, H2, Na, Na+, F? Explain. Order as many of... Problem 7ALQ: You are told that metal A is a better reducing agent than metal B. What, if anything, can be said... Problem 8ALQ: Explain the following relationships: G and w, cell potential and w, cell potential and G, cell... Problem 9ALQ: Explain why cell potentials are not multiplied by the coefficients in the balanced redox equation.... Problem 10ALQ: What is the difference between and ? When is equal to zero? When is equal to zero? (Consider regular... Problem 11ALQ: Consider the following galvanic cell: What happens to as the concentration of Zn2+ is increased? As... Problem 12ALQ: Look up the reduction potential for Fe3+ to Fe2+. Look up the reduction potential for Fe2+ to Fe.... Problem 13ALQ: If the cell potential is proportional to work and the standard reduction potential for the hydrogen... Problem 14ALQ: Is the following statement true or false? Concentration cells work because standard reduction... Problem 15RORR: Define oxidation and reduction in terms of both change in oxidation number and electron loss or... Problem 16RORR: Assign oxidation numbers to all the atoms in each of the following:... Problem 17RORR: Specify which of the following equations represent oxidation reduction reactions, and indicate the... Problem 18RORR: The Ostwald process for the commercial production of nitric acid involves the Following three steps:... Problem 19Q: What is electrochemistry? What are redox reactions? Explain the difference between a galvanic and an... Problem 20Q: When balancing equations in Chapter 3, we did not mention that reactions must be charge balanced as... Problem 21Q: When magnesium metal is added to a beaker of HCl(aq), a gas is produced. Knowing that magnesium is... Problem 22Q: How can one construct a galvanic cell from two substances, each having a negative standard reduction... Problem 23Q: The free energy change for a reaction, G, is an extensive property. What is an extensive property?... Problem 24Q: What is wrong with the following statement: The best concentration cell will consist of the... Problem 25Q: When jump-starting a car with a dead battery, the ground jumper should be attached to a remote part... Problem 26Q: In theory, most metals should easily corrode in air. Why? A group of metals called the noble metals... Problem 27Q: Consider the electrolysis of a molten salt of some metal. What information must you know to... Problem 28Q: Consider the following electrochemical cell: a. If silver metal is a product of the reaction, is the... Problem 29E Problem 30E Problem 31E: Balance the following oxidationreduction reactions that occur in basic solution. a.... Problem 32E: Balance the following oxidationreduction reactions that occur in basic solution. a.... Problem 33E: Chlorine gas was first prepared in 1774 by C. W. Scheele by oxidizing sodium chloride with... Problem 34E Problem 35E: Consider the following galvanic cell: Label the reducing agent and the oxidizing agent, and describe... Problem 36E: Consider the following galvanic cell: a. Label the reducing agent and the oxidizing agent, and... Problem 37E: Sketch the galvanic cells based on the following overall reactions. Show the direction of electron... Problem 38E: Sketch the galvanic cells based on the following overall reactions. Show the direction of electron... Problem 39E: Calculate values for the galvanic cells in Exercise 37. Problem 40E: Calculate values for the galvanic cells in Exercise 38. Problem 41E: Sketch the galvanic cells based on the following half-reactions. Show the direction of electron... Problem 42E: Sketch the galvanic cells based on the following half-reactions. Show the direction of electron... Problem 43E: Give the standard line notation for each cell in Exercises 37 and41. Problem 44E: Give the standard line notation for each cell in Exercises 38 and 42. Problem 45E: Consider the following galvanic cells: For each galvanic cell, give the balanced cell equation and... Problem 46E: Give the balanced cell equation and determine for the galvanic cells based on the following... Problem 47E: Calculate values for the following g cells. Which reactions are spontaneous as written (under... Problem 48E: Calculate values for the following cells. Which reactions are spontaneous as written (under standard... Problem 49E: Chlorine dioxide (C1O2), which is produced by the reaction 2NaC1O2(aq)+Cl2(g)2ClO2(g)+2NaCl(aq) has... Problem 50E: The amount of manganese in steel is determined by changing it to permanganate ion. The steel is... Problem 51E: Calculate the maximum amount of work that can be obtained from the galvanic cells at standard... Problem 52E: Calculate the maximum amount of work that can be obtained from the galvanic cells at standard... Problem 53E: Estimate for the half-reaction 2H2O+2eH2+2OH given the following values of Gfo: H2O(l)=237kJ/mol... Problem 54E: The equation G = nF also can be applied to half-reactions. Use standard reduction potentials to... Problem 55E: Glucose is the major fuel for most living cells. The oxidative breakdown of glucose by our body to... Problem 56E: Direct methanol fuel cells (DMFCs) have shown some promise as a viable option for providing green... Problem 57E: Using data from Table 18.1, place the following in order of increasing strength as oxidizing agents... Problem 58E: Using data from Table 18.1, place the following in order of increasing strength as reducing agents... Problem 59E: Answer the following questions using data from Table 17-1 (all under standard conditions). a. Is... Problem 60E: Answer the following questions using data from Table 17-1 (all under standard conditions). a. Is... Problem 61E: Consider only the species (at standard conditions) Na+, Cl, Ag+, Ag, Zn2+, Zn, Pb in answering the... Problem 62E Problem 63E: Use the table of standard reduction potentials (Table 17-1) to pick a reagent that is capable of... Problem 64E: Use the table of standard reduction potentials (Table 18.1) to pick a reagent that is capable of... Problem 65E Problem 66E Problem 68E: Consider the concentration cell in Fig. 17-10. If the Fe2+ concentration in the right compartment is... Problem 69E: Consider the concentration cell shown below. Calculate the cell potential at 25C when the... Problem 70E: Consider a concentration cell similar to the one shown in Exercise 69, except that both electrodes... Problem 71E: The overall reaction in the lead storage battery is Pb(s)+PbO2(s)+2H+(aq)+2HSO4(aq)2PbSO4(s)+2H2O(l)... Problem 72E: Calculate the pH of the cathode compartment for the following reaction given = 3.01 V when [Cr3+] =... Problem 73E: Consider the cell described below: Zn|Zn2+(1.00M)||Cu2+(l.00M)|Cu Calculate the cell potential after... Problem 74E: Consider the cell described below: Al|Al3+(1.00M)||Pb2+(1.00M)|Pb Calculate the cell potential after... Problem 75E: Calculate G and K at 25C for the reactions in Exercises 37 and 41. Problem 76E: Calculate G and K at 25C for the reactions in Exercises 38 and 42. Problem 77E: Consider the galvanic cell based on the following half-reactions: a. Determine the overall cell... Problem 78E: Consider the galvanic cell based on the following half-reactions: a. Determine the overall cell... Problem 79E: An electrochemical cell consists of a standard hydrogen electrode and a copper metal electrode. a.... Problem 80E Problem 81E: An electrochemical cell consists of a standard hydrogen electrode and a copper metal electrode. If... Problem 82E: An electrochemical cell consists of a nickel metal electrode immersed in a solution with [Ni2+] =... Problem 83E: Consider a concentration cell that has both electrodes made of some metal M. Solution A in one... Problem 84E: You have a concentration cell in which the cathode has a silver electrode with 0.10 M Ag+. The anode... Problem 85E: Under standard conditions, what reaction occurs, if any, when each of the following operations is... Problem 86E: A disproportionation reaction involves a substance that acts as both an oxidizing and a reducing... Problem 87E: Consider the following galvanic cell at 25C: Pt|Cr2+(0.30M),Cr3+(2.0M)||Co2+(0.20M)|Co The overall... Problem 88E: An electrochemical cell consists of a silver metal electrode immersed in a solution with [Ag+] = 1.0... Problem 89E Problem 90E: For the following half-reaction, = 2.07 V: A1F63(aq)+3eAl(s)+6F(aq) Using data from Table 17-1,... Problem 91E: Calculate for the following half-reaction: AgI(s)+eAg(s)+I(aq) (Hint: Reference the Ksp value for... Problem 92E: The solubility product for CuI(s) is 1.1 102 Calculate the value of for the half-reaction... Problem 93E: How long will it take to plate out each of the following with a current of 100.0 A? a. 1.0 kg AI... Problem 94E: The electrolysis of BiO+ produces pure bismuth. How long would it take to produce 10.0 g Bi by the... Problem 95E: What mass of each of the following substances can be produced in 1.0 h with a current of 15 A? a. Co... Problem 96E: Aluminum is produced commercially by the electrolysis of Al2O3 in the presence of a molten salt. If... Problem 97E: An unknown metal M is electrolyzed. It took 74.1 s for a current of 2.00 A to plate out 0.107 g of... Problem 98E Problem 99E: What volume of F2 gas, at 25C and 1.00 atm, is produced when molten KF is electrolyzed by a current... Problem 100E: What volumes of H2(g) and O2(g) at STP are produced from the electrolysis of water by a current of... Problem 101E: A single HallHeroult cell (as shown in Fig. 17-22) produces about 1 ton of aluminum in 24 h. What... Problem 102E: A factory wants to produce 1.00 103 kg barium from the electrolysis of molten barium chloride. What... Problem 103E: It took 2.30 min using a current of 2.00 A to plate out all the silver from 0.250 L of a solution... Problem 104E: A solution containing Pt4+ is electrolyzed with a current of 4.00 A. How long will it take to plate... Problem 105E: A solution at 25C contains 1.0 M Cd2+, 1.0 M Ag+, 1.0 M Au3+, and 1.0 M NiH2+ in the cathode... Problem 106E Problem 107E: In the electrolysis of an aqueous solution of Na2SO4, what reactions occur at the anode and the... Problem 108E: Copper can be plated onto a spoon by placing the spoon in an acidic solution of CuSO4(aq) and... Problem 109E Problem 110E Problem 111E: What reactions take place at the cathode and the anode when each of the following is electrolyzed?... Problem 112E: What reaction will take place at the Cathode and the anode when each of the following is... Problem 113AE: Gold is produced electrochemically from an aqueous solution of Au(CN)2 containing an excess of CN.... Problem 114AE: The blood alcohol (C2H5OH) level can be determined by titrating a sample of blood plasma with an... Problem 115AE: The saturated calomel electrode. abbreviated SCE. is often used as a reference electrode in making... Problem 116AE: Consider the following half-reactions: Explain why platinum metal will dissolve in aqua regia (a... Problem 117AE: Consider the standard galvanic cell based on the following half-reactions: Cu2++2eCuAg++eAg The... Problem 118AE: A standard galvanic cell is constructed so that the overall cell reaction is... Problem 119AE: The black silver sulfide discoloration of silverware can be removed by heating the silver article in... Problem 120AE Problem 121AE: When aluminum foil is placed in hydrochloric acid, nothing happens for the first 30 seconds or so.... Problem 122AE Problem 123AE: A fuel cell designed to react grain alcohol with oxygen has the following net reaction:... Problem 124AE: The overall reaction and equilibrium constant value for a hydrogen-oxygen fuel cell at 298 K is... Problem 125AE Problem 126AE: The overall reaction and standard cell potential at 25C for the rechargeable nickel-cadmium alkaline... Problem 127AE Problem 128AE: The ultimate electron acceptor in the respiration process is molecular oxygen. Electron transfer... Problem 129AE: One of the few industrial-scale processes that produce organic compounds electrochemically is used... Problem 130AE: It took 150. s for a current of 1.25 A to plate out 0.109 g of a metal from a solution containing... Problem 131AE Problem 132AE: In the electrolysis of a sodium chloride solution, what volume of H2(g) is produced in the same time... Problem 133AE: An aqueous solution of an unknown salt of ruthenium is electrolyzed by a current of 2.50 A passing... Problem 134CWP: Which of the following statement(s) is/are true? a. Copper metal can be oxidized by Ag+ (at standard... Problem 135CWP: Consider a galvanic cell based on the following half-reactions: a. What is the expected cell... Problem 136CWP Problem 137CWP: Consider a galvanic cell based on the following half-reactions: a. What is the standard potential... Problem 138CWP: An electrochemical cell consists of a silver metal electrode immersed in a solution with [Ag+] =... Problem 139CWP: An aqueous solution of PdCl2 is electrolyzed for 48.6 seconds, and during this time 0.1064 g of Pd... Problem 140CP Problem 141CP Problem 142CP: The overall reaction in the lead storage battery is Pb(s)+PbO2(s)+2H+(aq)+2HSO4(aq)2PbSO4(s)+2H2O(l)... Problem 143CP: Consider the following galvanic cell: Calculate the KSP value for Ag2SO4(s). Note that to obtain... Problem 144CP: A zinc-copper battery is constructed at follows at 25C: Zn|Zn2+(0.10M)||Cu2+(2.50M)|Cu The mass of... Problem 145CP: A galvanic cell is based on the following half-reactions: where the iron compartment contains an... Problem 146CP: Consider a cell based on the following half-reactions: a. Draw this cell under standard conditions,... Problem 147CP Problem 148CP: You have a concentration cell with Cu electrodes and [Cu2+] = 1.00 M (right side) and 1.0 104M... Problem 149CP: A galvanic cell is based on the following half-reactions: In this cell, the silver compartment... Problem 150CP: Given the following two standard reduction potentials, solve for the standard reduction potential of... Problem 151CP: Consider the following galvanic cell: Calculate the concentrations of Ag+(aq) and Ni2+(aq) once the... Problem 152CP Problem 153CP: Consider the following galvanic cell: A 15 0-mole sample of NH is added to the Ag compartment... Problem 154CP: When copper reacts with nitric acid, a mixture of NO(g) and NO2(g) is evolved. The volume ratio of... Problem 155IP: The following standard reduction potentials have been determined for the aqueous chemistry of... Problem 156IP: An electrochemical cell is set up using the following unbalanced reaction: Ma+(aq)+N(s)N2+(aq)+M(s)... Problem 157IP: Three electrochemical cells were connected in series so that the same quantity of electrical current... Problem 158IP: A silver concentration cell is set up at 25C as shown below: The AgCl(s) is in excess in the left... Problem 159MP: A galvanic cell is based on the following half-reactions: In this cell, the copper compartment... Problem 160MP: The table below lists the cell potentials for the 10 possible galvanic cells assembled from the... Problem 1RQ: What is a half-reaction? Why must the number of electrons lost in the oxidation half reaction equal...
Related questions
Concept explainers
1A. Identify the species getting oxidized or reduced, as well as the oxidizing and reducing agents in the following reactions.
Mn2+ + BiO3 → MnO4 - + Bi3+
Oxidizing agent: __________________
Reducing agent: __________________
Reduced: __________________
1B.
The following are isomers of each other.
I. pentane
II. 2,2,-dimethylpropane
III. 2-methylbutane
A. Alkanes are nonpolar.
B. Alkanes are non-flammable.
C. Every carbon in an alkane has four bonds.
D. Alkanes contain only C and H atoms.
E. Alkanes do not contain carbon-carbon double or triple bonds.
1C.
What is the correct name for (picture is attatched)
A. 3-ethyl-2-methylbutane
D. 1,2,3-trimethylpropane
Definition Definition Simplest organic compounds that contain only single bonds between carbon – carbon atoms and carbon – hydrogen bonds. This means they are a series of saturated compounds with a general molecular formula, C n H 2n+2 .
Expert Solution
Oxidized species will release electrons.
Reduced species will gain electrons.
Oxidizing species will oxidized another substance therefore itself gets reduced.
Reducing species will reduced another substance therefore itself gets oxidized.
1A :
Mn here releases electron and goes from +2 to +7 oxidation state. So ,
BiO3 - will accept electron and goes from +5 to +3 oxidation state.
Oxidized : MnO4 -
Oxidizing agent: BiO3 -
Reducing agent: Mn(II)
Step by step
Solved in 4 steps with 2 images
UNLOCK THE REST