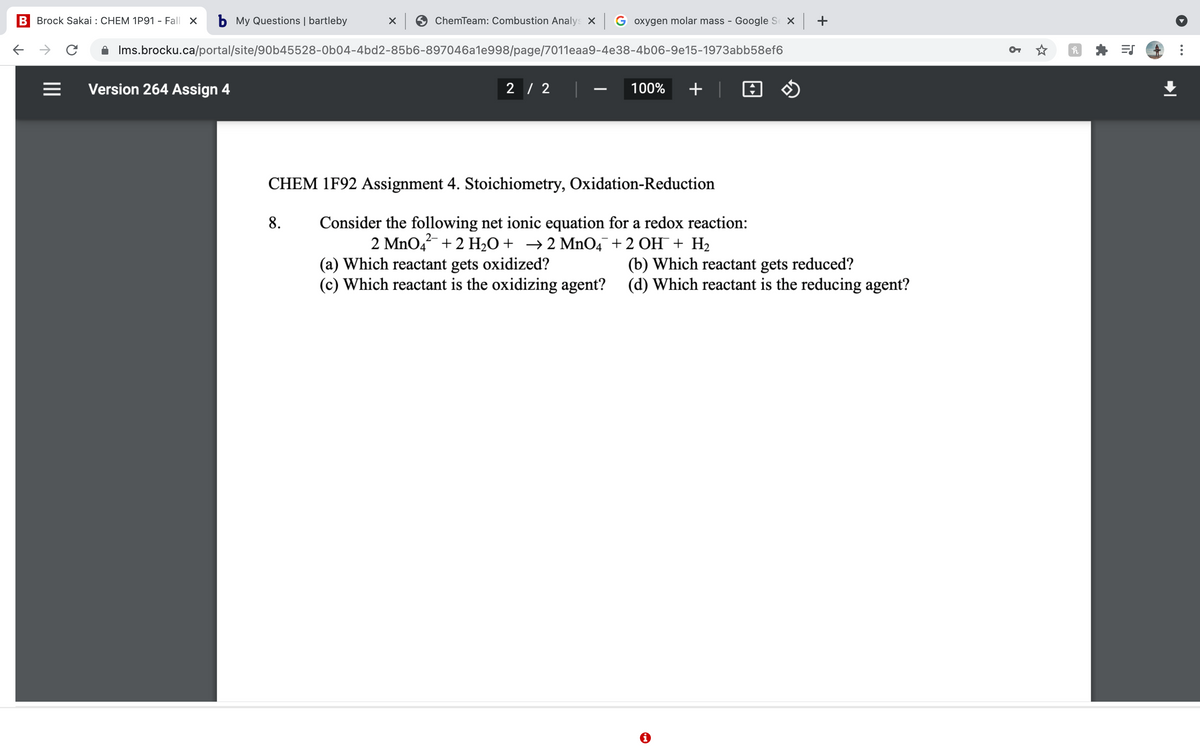
1F92 Assignment 4. Stoichiometry, Oxidation-Reduction Consider the following net ionic equation for a redox reaction: 2 MnO,- +2 H2O + →2 MnO4¯ +2 OH + H2 (a) Which reactant gets oxidized? (c) Which reactant is the oxidizing agent? (d) Which reactant is the reducing agent? (b) Which reactant gets reduced?
1F92 Assignment 4. Stoichiometry, Oxidation-Reduction Consider the following net ionic equation for a redox reaction: 2 MnO,- +2 H2O + →2 MnO4¯ +2 OH + H2 (a) Which reactant gets oxidized? (c) Which reactant is the oxidizing agent? (d) Which reactant is the reducing agent? (b) Which reactant gets reduced?
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section11.6: Properties Of Liquids
Problem 1.2ACP
Related questions
Question
Transcribed Image Text:B Brock Sakai : CHEM 1P91 - Fall X
b My Questions | bartleby
ChemTeam: Combustion Analys X G oxygen molar mass - Google S X +
A Ims.brocku.ca/portal/site/90b45528-0b04-4bd2-85b6-897046a1e998/page/7011eaa9-4e38-4b06-9e15-1973abb58ef6
Version 264 Assign 4
2 / 2
100%
+ |
CHEM 1F92 Assignment 4. Stoichiometry, Oxidation-Reduction
Consider the following net ionic equation for a redox reaction:
2 MnO4 + 2 H2O+ →2 Mn04¯+2 OH¯+ H2
8.
(a) Which reactant gets oxidized?
(c) Which reactant is the oxidizing agent? (d) Which reactant is the reducing agent?
(b) Which reactant gets reduced?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning