Science behind corrosion-test
Corrosion is defined as an activity that transforms refined metals into more chemically stable forms such as oxide, hydroxide, carbonate, or sulfide. It refers to the slow decomposition of things (typically metals); thanks to chemical and/or electrochemical reactions with their surroundings. Corrosion engineering is the science of preventing and controlling corrosion.
Corrosion
Corrosion is defined as an activity that transforms refined metals into more chemically stable forms such as oxide, hydroxide, carbonate, or sulfide. It refers to the slow decomposition of things (typically metals); thanks to chemical and/or electrochemical reactions with their surroundings. Corrosion engineering is the science of preventing and controlling corrosion.
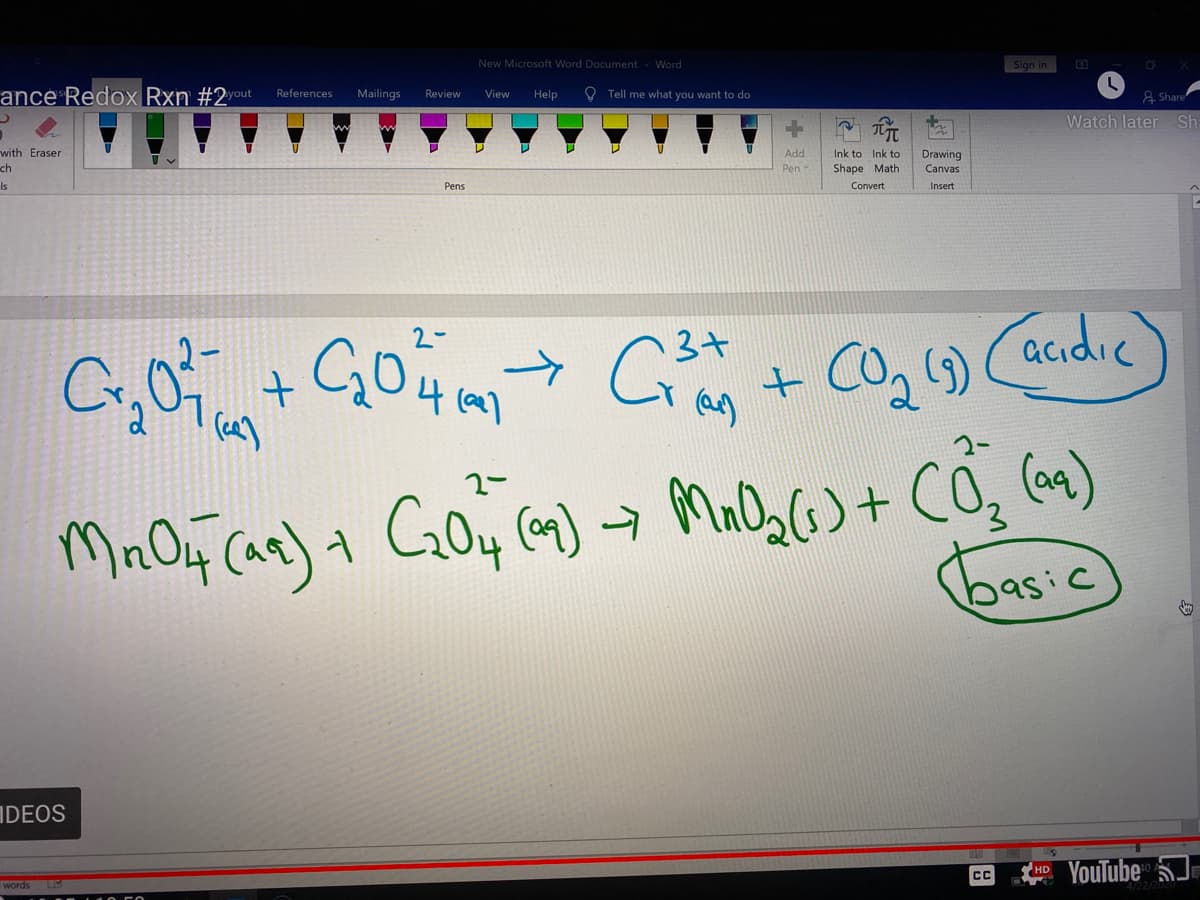
a) The reaction given is,
=> Cr2O72- (aq) + C2O42- (aq) --------> Cr3+ (aq) + CO2 (g)
In the above reaction, the oxidation state of Cr initially in Cr2O72- is 6+ (because each O is in 2+ oxidation state and there is 2- charge) and finally its in 3+ oxidation state.
And C is initially in 3+ oxidation state in C2O42- and finally its in 4+ oxidation state in CO2
Hence the oxidation of C in C2O42- and reduction of Cr in Cr2O72- is taking place in the reaction.
Hence the half reactions taking place can be written as,
Oxidation : C2O42- (aq) -------> CO2 (g) + e-
Balancing : Since we have 2 C in LHS. Hence making it 2 in RHS also.
=> C2O42- (aq) -------> 2 CO2 (g) + e-
Now we have 2- charge in LHS. Hence making it 2 - in RHS also.
=> C2O42- (aq) -------> 2 CO2 (g) + 2 e-
Since both number of each elements and the charge is equal in both side of the reaction now.
Hence the reaction is now balanced.
Reduction : Cr2O72- (aq) + e- ------> Cr3+ (aq)
Balancing : Since we have 2 Cr in LHS. Hence making it 2 in RHS also.
=> Cr2O72- (aq) + e- ------> 2 Cr3+ (aq)
Now we have 7 O in LHS. Hence balancing it in RHS using water.
=> Cr2O72- (aq) + e- ------> 2 Cr3+ (aq) + 7 H2O (l)
Now we have 14 H in the RHS. Hence balancing it in LHS using proton because the medium is acidic.
=> Cr2O72- (aq) + 14 H+ (aq) + e- ------> 2 Cr3+ (aq) + 7 H2O (l)
Since total charge in RHS is 6+. Hence making it 6+ in LHS also.
=> Cr2O72- (aq) + 14 H+ (aq) + 6 e- ------> 2 Cr3+ (aq) + 7 H2O (l)
Since both number of each elements and the charge is equal in both side of the reaction now.
Hence the reaction is now balanced.
Multiplying the oxidation half reaction with 3 and then adding it with reduction half reaction to get overall balanced redox reaction by cancelling 6 electrons as,
=> Cr2O72- (aq) + 14 H+ (aq) + 3 C2O42- (aq) -------> 6 CO2 (g) + 2 Cr3+ (aq) + 7 H2O (l)
Step by step
Solved in 8 steps




