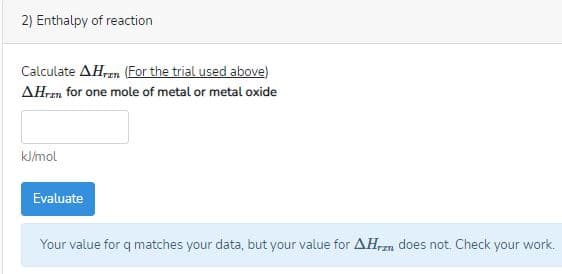
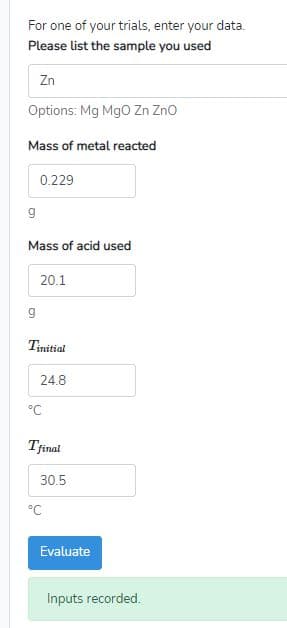
2) Enthalpy of reaction Calculate AH,n (For the trial used above) AHren for one mole of metal or metal oxide kJ/mol Evaluate Your value for q matches your data, but your value for AH,rzn does not. Check your work.
Q: The 1995 Nobel Prize in Chemistry was shared by Paul Crutzen, F. Sherwood Rowland, and Mario Molina…
A: Given: (1) ClO(g)+O3(g)⟶Cl(g)+2O2(g)ΔH∘rxn=−122.8 kJ (2) 2O3(g)⟶3O2(g)ΔH∘rxn=−285.3 kJ (3)…
Q: The 1995 Nobel Prize in Chemistry was shared by Paul Crutzen, F. Sherwood Rowland, and Mario Molina…
A: According to Hess law, if a reaction undergoes in multiple stages, the enthalpy change of the…
Q: Solve, given delta H.
A: The given equations are :
Q: Using the given molar enthalpies of formation, determine the change in enthalpy for the combustion…
A: We have to calculate the change in enthalpy for the given reaction
Q: From the following enthalpies of reaction: H2(g)+F2(g)→2HF(g),ΔH=−537kJ…
A: H2(g)+f2(g)-> 2hf(g) triangle h=-537 kj C(S)+2f2 (g) àcf4 (g) triangle h=-680 kj eqn2…
Q: Given the following standard enthalpies; H2(g) ➡ 2H(g) △H = +436KJ O2(g) ➡…
A: The two reactions given are, 1) H2 (g) --------> 2 H (g) ΔH =…
Q: From the following enthalpies of reaction find ΔHrxn for 2 HCl(g) + F2(g) ---> 2 HF(l) + Cl2(g): 4…
A: Given, 4 HCl(g) + O2(g) ---> 2 H2O(l) + 2 Cl2(g); ΔH = -148.4 kJ/mol1/2 H2(g) + 1/2 F2(g) --->…
Q: Calculate the enthapy AH for the reaction of: C2H2(g) + O29) - CO219) + H2O0) Note: Balance the…
A: The question deals with thermochemistry. The amount of energy absorbed or released during a chemical…
Q: Bond Average Bond Enthalpy (kJ/mol) N-H 391 H-H 436 N= N ? (b) Based on the average bond enthalpies…
A: Answer: In this question with the help of standard enthalpy of formation of ammonia and given bond…
Q: 2. Appropriately combine the measured enthalpies of reactions (2) and (3) to predict AH, for the…
A: Since you have posted multiple questions, the answer for first question is given below. Kindly…
Q: How do the three reactions relate to each other? Also, how do the given enthalpy values relate to…
A: Enthalpy of the first two reactions can be used to calculate the enthalpy of the third reactionThe…
Q: (d) Calculate the average bond enthalpy for the N-H bond using the following data: NH₂(g) → NH₂(g) +…
A:
Q: B. AG = AH -TAS D. E mc2 4. What do you need to consider when looking for enthalpy value of a…
A:
Q: Compute for the standard enthalpy change of the reaction: 2H,Se + 2H,O → 2H,O@+ SO2(2) (g) Given the…
A:
Q: Chapter 6 : Energy relationship in chemical rxn B Example : A 0.1375g sample solid of Mg (M.w=24.31)…
A: Given mass of Mg = 0.1375 g Molar mass of Mg = 24.31 g/mole Heat capacity of the calorimeter =…
Q: The 1995 Nobel Prize in Chemistry was shared by Paul Crutzen, F. Sherwood Rowland, and Mario Molina…
A:
Q: Given the following standard molar enthalpy of formation (DHof): (D = delta)…
A: Given, 2CH3OH(l) + 3O2(g) --> 2CO2(g) + 4H2O(l)
Q: Using the equations H, (g) + F, (g) → 2 HF (g) AH° = -79.2 kJ/mol C'(s) + 2 F, (g) → CF, (g) AH° =…
A: Given, Equation1: H2(g) + F2(g) 2HF(g) ∆H = -79.2 KJ/mol Equation2: C(s)…
Q: Estimate the reaction enthalpy for the gas phase reaction of elemental hydrogen (H2) and elemental…
A:
Q: Use the data found in Thermodynamic Properties and the data in Bond Energies. to estimate the…
A: Bond Bond energy ( kJ/ mol) N- F 272 N- H 391 N-N 160 C-F 485 C-C 347 C-H 413…
Q: Using the provided table, determine the molar enthalpy (in kJ/mol) for the reaction 2 NH, (g) + 3…
A:
Q: Consider the following reaction: CO2 (g) + 4H2(g) —> CH4 (g) + 2H2O (g). CO2 (g): -384.60 kJ/mol…
A: To solve this problem we have to calculate the enthalpy change of the given reaction .
Q: Given the following reaction enthalpies: P,(s) + 6 Cl,(g) → 4 PCI,(g) =-1225.6moi → PO,(s) → PCI,(g)…
A:
Q: 15 Calculate ΔGrxn in (kJ/mol) for the reaction for which ΔHrxn=-26.1kJ/mol ; ΔS=3.89J/molK at…
A: Gibb's Free energy isbthe change of free energy during a reaction. It can be positive negative or…
Q: Calculate the bond energy per mole for forming all the bonds of carbon dioxide, CO2. Express your…
A: Structure of CO2 is O=C=O There are two C=O bonds in CO2 The energy required for the formation of…
Q: Question attached
A: Given: The given reaction is
Q: Use the given standard enthalpies of formation to determine the heat of reaction of the following…
A: Dear student , since you have posted multiple parts questions we will allow to solve only first…
Q: 6.62 From the following data, TARIS → CO2(g) AHan = -393.5 kJ/mol C(graphite) + O2(g) H,(g) + 0,(g)…
A: Hess's law states that the enthalpy change for a reaction is the sum of the enthalpy changes of the…
Q: Determine the AS° for the reaction: H2SO4(L) 156.9 H2OL) SO3(2) 256.2 S° 69.9
A: The standard entropy change ∆S∘ for the reaction can be calculated from the following equation:…
Q: 5. The chemistry of nitrogen oxides is very versatile. Given the following reactions and their…
A:
Q: Calculate the standard enthalpy change for the reaction 2A +B=20 + 2D Use the following data: AH…
A: The enthalpy change can be calculated via subtracting that of reactants from that of products.
Q: Question attached
A: Given EBr-Br = 193 kJ/molEF-F = 155 kJ/molEBr-F = 237 kJ/mol Balance equation : Br2 + 3F2 →…
Q: Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab,…
A: Solution -
Q: The 1995 Nobel Prize in Chemistry was shared by Paul Crutzen, F. Sherwood Rowland, and Mario Molina…
A: Enthalpy change follows simple algebraic rules in reactions according to Hess law. Thus, if a…
Q: Calculate the amount of milk at 4°C needed to lower the temperature of 250 mL of coffee at 95°C to…
A:
Q: Using the enthalpy of reaction for two reactions with ozone, determine the enthalpy of reaction for…
A: To determine the enthalpy of reaction for the reaction of chlorine with ozone. we first derive the…
Q: Brz (CHs)a COH, heat 2. 1. light 2.1) Os ; i) Me ,s' Cs Hio O Na OCHs CH30H, heat 3.
A: Organic chemistry involves a vast collection of oxidizing and reducing agents. The type of base…
Q: Is the heat of Mg combustion equal to the heat of formation of MgO when both are measured at…
A:
Q: Hydrogenation of double and triple bonds is an important industrial process. Calculate (in kJ) the…
A: Introduction- To determine the standard enthalpy change ΔH° ( in KJ ) for the hydrogenation of…
Q: i) Balance the equation below for the formation of one mole of ammonia, NH3, from its elements.…
A: Mean Bond energy of H-H = 436 kJ/mol Mean Bond energy of C-C = 348 kJ/mol Mean Bond energy of N-N =…
Q: 3. Use the appropriate data from the table to calculate the change in enthalpy for this reaction:…
A:
Q: Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab,…
A: Standard enthapies H2S = -20.6 KJ/mol O2 = 0 S8 = 0 H2O = - 258.8 KJ/mol ∆Hrxn = ?
Q: The enthalpy of reaction for the formation of 2.00-mol ozone from 3.00-mol O2 (g): 3 O2 (g) à 2 O3…
A: The reaction given is => 3 O2 (g) ------> 2 O3 (g)…
Q: The standard molar enthalpy of formation for oxygen gas, ?2(?), is zero. The enthalpy of formation…
A: Enthalpy of formation is enthalpy change or energy change that occurs when one mole of compound is…
Q: OWLV2 | Online teaching and lear X b Login bartleby x G Given the standard enthalpy char x +…
A: We have to find specific heat capacity for Ag.
Q: Pentaborane, Bs Ha, was once i Studied asYatpotentials roCket fuel.)calculate Srutthe heat given…
A:
Q: Use the given standard enthalpies of formation to calculate AH° for the following reaction (SHOW…
A:
Q: Calculate the standard enthalpy change for the reaction 2A +Bu 20 + 2D where the heats of formation…
A: The reaction taking place is given as, => 2 A + B -----> 2 C + 2 D And the standard formation…
Q: Given the following processes and their respective enthalpies in kJ/mol: Li(s) -->…
A:
How do you solve for Hrxn?
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

- A 50.00mL sample with BaCl2 was diluted to 650 mL. Aliquots of 100.00 mL of this solution were analysed using Mohr and Volhard methods. The following data were obtained. Volhard method: Volume of AgNO3 = 100mL; Volume of KSCN = 18.25mL Mohr Method: volume of AgNO3: sample = 27.80mL (blank) = 0.30mLWhat is the %BaCl2 using Mohr and Volhard Method. Concentration of KSCN: 0.04000M Concentration of AgNO3: 0.03000MWhy is glass better than fused silica as a prism construction material for a monochromator to be used in the region of 400 to 80 nm?b. Given that the cuvette path length was 1.17 cm, use the slope of this line to calculate the molar absorptivity of the cobalt complex at this wavelength. (Assume Beer’s law is obeyed.) c. An unknown cobalt sample was treated as follows: 40.8 mg of the unknown was dissolved in solvent to prepare 50.00 mL of solution in a volumetric flask. 4.00 mL of this solution was treated with thiocyanate, and diluted to a final volume of 50.00 mL. A sample of this final solution was placed in the same cuvette as the standards, and showed an absorbance of 0.38 at 625 nm. Determine the percentage of cobalt in the original unknown, given that the atomic mass of cobalt is 58.933 g/mol.
- For bulk crystalline sample which technique is best ? Give me handwritten answer with explaination?you've been tasked to compound 30 grams of a 0.5% Zinc oxide ointment. you have a stock preparation of zinc oxide 5% strength to mix with petrolatum to dilute the ordered strength. In what (parts) ratio could this be compounded?(d) By using the data provided below, calculate the PillingBedworth ratio for the nickel (Ni) – nickel oxide (NiO) system and determine whether NiO is protective against corrosion. Atomic mass of nickel = 58.7 g.mol-1 Density of nickel = 8.91 Mg.m-3 Molecular mass of nickel oxide = 74.7 g.mol-1 Density of nickel oxide = 6.67 Mg.m-3 (e) A stainless steel pressure vessel that has fracture toughness, K1C, of 197 MN.m-3/2 is going to be exposed to a stress of 398 MN.m-2 . By using this information explain how designers can determine the thickness of the vessel so that catastrophic failure can be avoided.
- In general, we want to have absorptions less than or equal to 1. Given that an ethanol solution of compound Y (150.0 g/mol) in a 1.00 cm quartz cell has an extinction coefficient (ε) of 23,150 at λmax=235 nm, what is the top concentration, in μM, we should consider for compound Y? Report your answer to the correct number of significant figures. Do not report the units in your answer.What are the conditions for a successful gravimetric analysis. Define nucleation. What conditions are important for this stage.A solution of altura red of concentration 307 ppm gives an absorbance of 0.616. What concentration is a solution of allura red which gives an absorbance reading of 0.261? The same cell (cuvette) with a path length of 1.000 cm is used for both readings. Report your answer to the nearest whole number without units? Hint: A=abc, where c can have any concentration units. dont provide handwriten solution
- From the study with UV-Vis spectroscopy, four samples of solutions with different known concentrations of atrazine were analyzed, using a 1 cm optical path cuvette at 220 nm. The analytical curve (calibration curve) with absorbance values (y-axis) per atrazine concentration (x-axis) provided a straight line with the following parameters: linear coefficient = 0 and slope = 760. assumptions of the Lambert-Beer Law, evaluate the following statements: I. The molar absorptivity coefficient (E), under the described experimental conditions, cannot be determined. II. A sample of a solution containing atrazine with an absorbance of 0.2 will show a concentration of 2.6x10-4 mol/L. III. Atrazine concentrations could be determined due to absorption taking place in the visible region. IV. The change in absorbance as a function of the analyte concentration is directly related to the absorptivity coefficient of atrazine and the length of the optical path of the cuvette. It is correct only what is…One common way to determine phosphorous in urine is to treat the sample after removing the protein with molybdenum (VI) and then reducing the resulting 12-molybdophosphate complex with ascorbic acid to give an intense blue-coloured species called molybdenum blue. The absorbance of molybdenum blue can be measured at 650 nm. A 24-hour urine sample was collected, and the patient produced 1122 mL in 24 hours. A 1.00 mL aliquot of the sample was treated with Mo (VI) and ascorbic acid and diluted to a volume of 50.00 mL A calibration curve was prepared by treating 1.00 mL aliquot of phosphate standard solution in the same manner as the urine sample. The absorbances of the standards and the urine sample were obtained at 650 nm and the following results were obtained. ppm, P aBSORBANCE (A) 1.003.00 0.230 2.00 0.436 3.00 0.638 4.00 0.848 Urine sample 0.518 ffind the slope and intercept, then graph it.Is the Cu detectable at a concentration of 0.10 mg/L in Flame-AAS? Please use data given.

