2) Fill in the blank by selecting one of the options below: a. Primary structure. b. Secondary structure. c. Tertiary structure. d. Quaternary structure :"amino acids are linked by peptide bonds to form a polypeptide chain." :"the structure formed by hydrogen bonds between hydrogen and oxygen atoms of the peptide backbone." : "The spatial arrangement of various tertiary structures." "It gives rise to two major molecular shapes called fibrous and globular." Mark the following True (T) or False (F) about enzymatic classification: H) Oxidoreductases: Transfer of electrons (hydride ions or H atoms). It catalyzes Oxidation-reduction reactions. ) Isomerases: Transfer of groups with molecules to yield isomeric form
2) Fill in the blank by selecting one of the options below: a. Primary structure. b. Secondary structure. c. Tertiary structure. d. Quaternary structure :"amino acids are linked by peptide bonds to form a polypeptide chain." :"the structure formed by hydrogen bonds between hydrogen and oxygen atoms of the peptide backbone." : "The spatial arrangement of various tertiary structures." "It gives rise to two major molecular shapes called fibrous and globular." Mark the following True (T) or False (F) about enzymatic classification: H) Oxidoreductases: Transfer of electrons (hydride ions or H atoms). It catalyzes Oxidation-reduction reactions. ) Isomerases: Transfer of groups with molecules to yield isomeric form
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
Please help with my biochem questions
Transcribed Image Text:pH
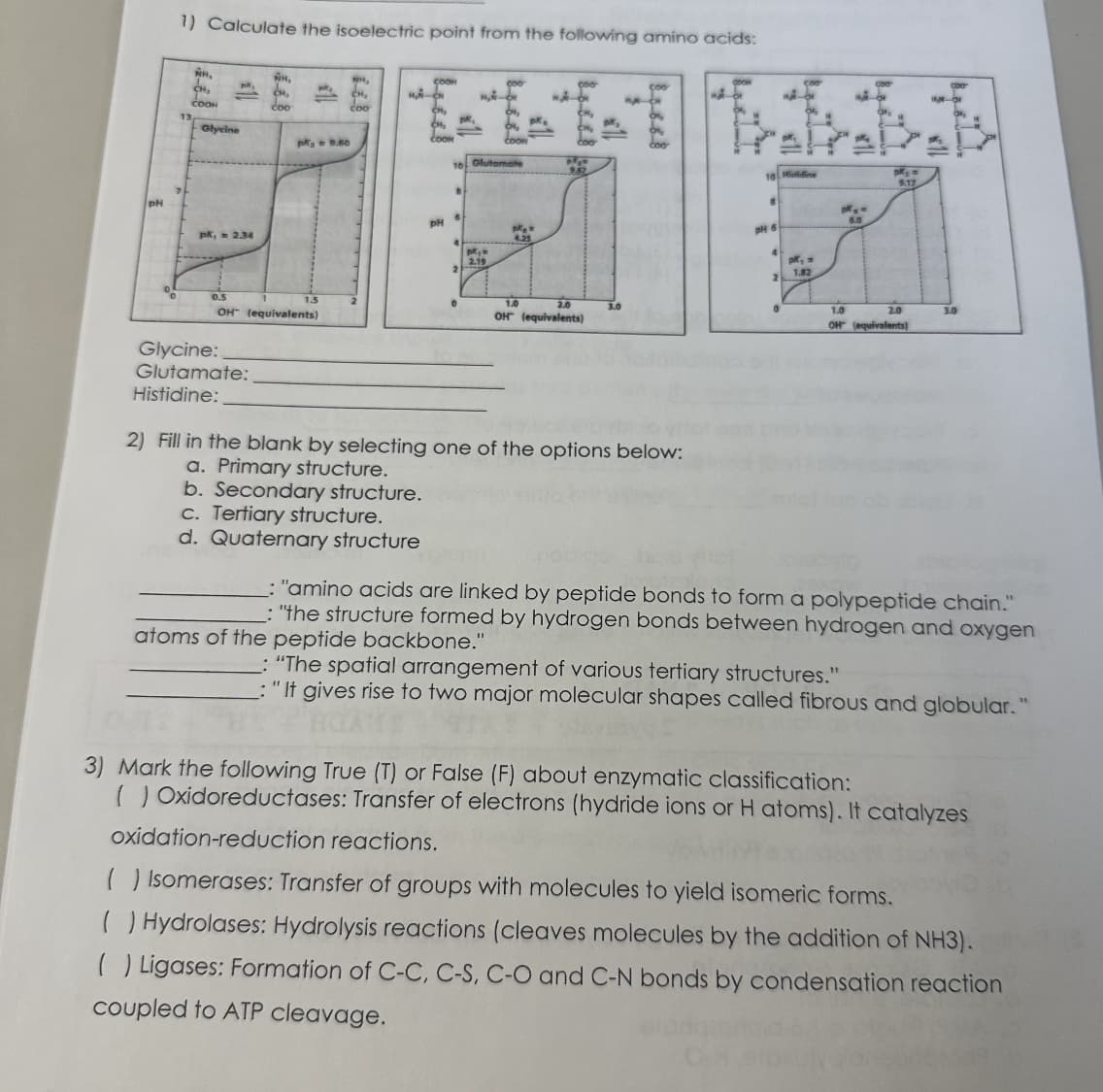
1) Calculate the isoelectric point from the following amino acids:
7
NR.
CH₂
COOH
Glycine
pk, 2.34
H₂
CH₂
coo
Glycine:
Glutamate:
Histidine:
pk = 9.60
OH (equivalents)
NH₂
CH₂
coo
COOK
MA-
CM₂
CH₂
COOH
PH
pk,
B
10 Glutamate
6
0
M₂-C
pk,
2.19
1 pk.
Coom
2) Fill in the blank by selecting one of
a. Primary structure.
b. Secondary structure.
c. Tertiary structure.
d. Quaternary structure
pk =
4.25
pk₂n
9.67
1.0
2.0
OH (equivalents)
3.0
options below:
Pretratat
18 Hiidine
8
pH 6
4
2
0
pk, =
1.82
pM₂ =
6.0
9.17
1.0
OH (equivalents)
2.0
1.
3.0
:"amino acids are linked by peptide bonds to form a polypeptide chain."
:"the structure formed by hydrogen bonds between hydrogen and oxygen
atoms of the peptide backbone."
"The spatial arrangement of various tertiary structures."
"It gives rise to two major molecular shapes called fibrous and globular."
3) Mark the following True (T) or False (F) about enzymatic classification:
( Oxidoreductases: Transfer of electrons (hydride ions or H atoms). It catalyzes
oxidation-reduction reactions.
( ) Isomerases: Transfer of groups with molecules to yield isomeric forms.
( ) Hydrolases: Hydrolysis reactions (cleaves molecules by the addition of NH3).
( ) Ligases: Formation of C-C, C-S, C-O and C-N bonds by condensation reaction
coupled to ATP cleavage.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON