2) If an unknown Cu*2 solution has an absorbance of .62, a) Find its molarity graphically: A M b) Using the equation of a line: y = mx + b for Beer's Law, A = (slope)M + 0 So, M = A/slope Measure the slope from your graph:
2) If an unknown Cu*2 solution has an absorbance of .62, a) Find its molarity graphically: A M b) Using the equation of a line: y = mx + b for Beer's Law, A = (slope)M + 0 So, M = A/slope Measure the slope from your graph:
Chapter22: Bulk Electrolysis: Electrogravimetry And Coulometry
Section: Chapter Questions
Problem 22.6QAP
Related questions
Question
How would I do question 2 given the graph I drew and the experimental data shown? Please do part a and part b. Thank you.
![Absorbance
:-
Beer's Law
Beer's Law Plot
Beer's Law Plot
1.40
yob
1.20
1.00
0.80
0.60
0.40
Absorbance
0.20
0.00
.02
.04
.06
.08
.1
Concentration (M)
Experimental Data:
[Cu*2]
.100 M
Absorbance
1.20
.080 M
0.97
.060 M
0.70
M
.040 M
0.50
.020 M
0.25
[Cu*2] unknown: Method a =
Method b =
.000 M
0.00](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F0ea55b65-6a30-46ce-96b8-2b732a599bb3%2F6210496d-5d72-4a54-98ac-ba599032c0f0%2Fjnqs3z2.jpeg&w=3840&q=75)
Transcribed Image Text:Absorbance
:-
Beer's Law
Beer's Law Plot
Beer's Law Plot
1.40
yob
1.20
1.00
0.80
0.60
0.40
Absorbance
0.20
0.00
.02
.04
.06
.08
.1
Concentration (M)
Experimental Data:
[Cu*2]
.100 M
Absorbance
1.20
.080 M
0.97
.060 M
0.70
M
.040 M
0.50
.020 M
0.25
[Cu*2] unknown: Method a =
Method b =
.000 M
0.00
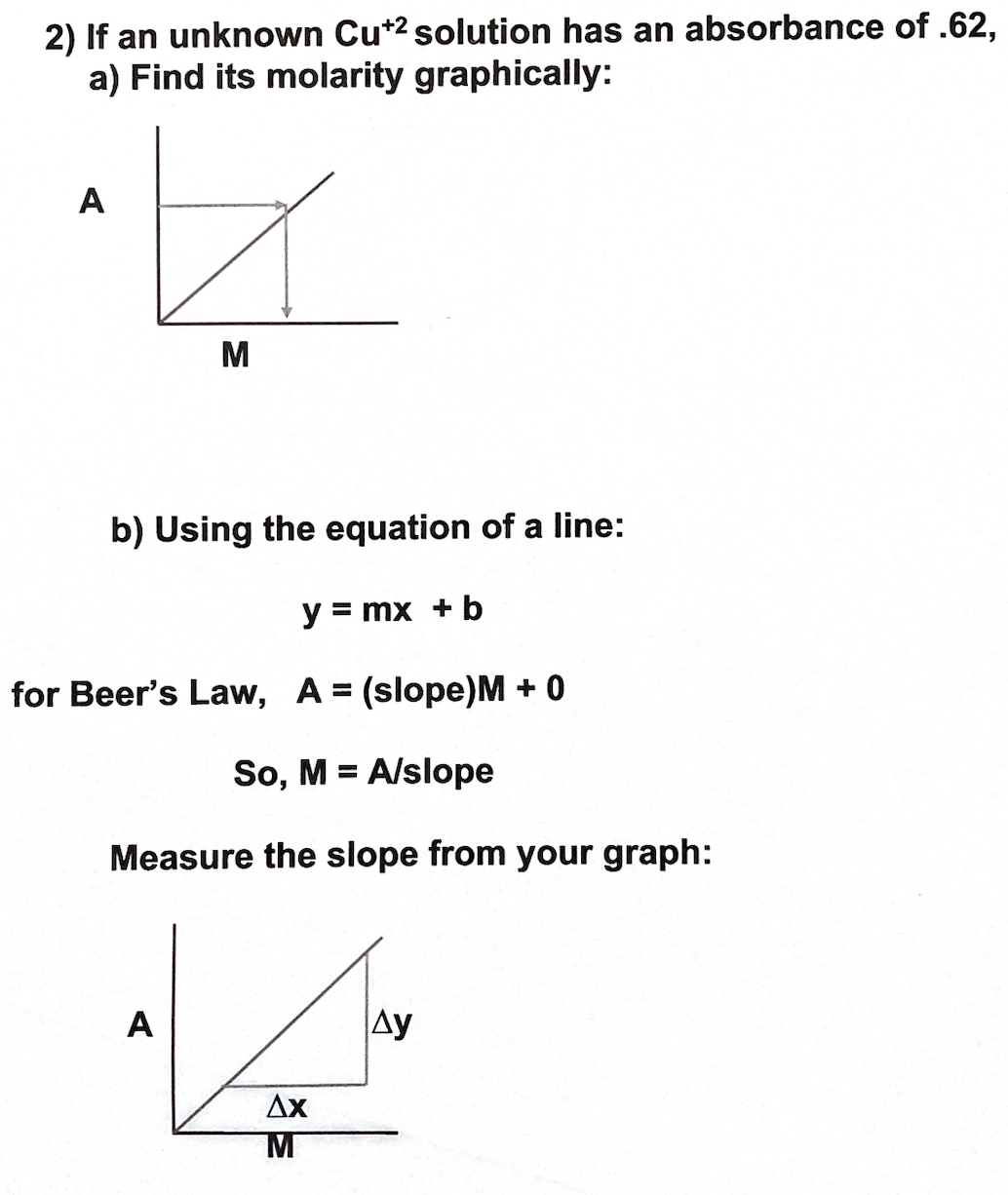
Transcribed Image Text:2) If an unknown Cu*2 solution has an absorbance of .62,
a) Find its molarity graphically:
A
M
b) Using the equation of a line:
y = mx + b
for Beer's Law, A = (slope)M + 0
So, M = A/slope
Measure the slope from your graph:
A
Ay
Ax
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

