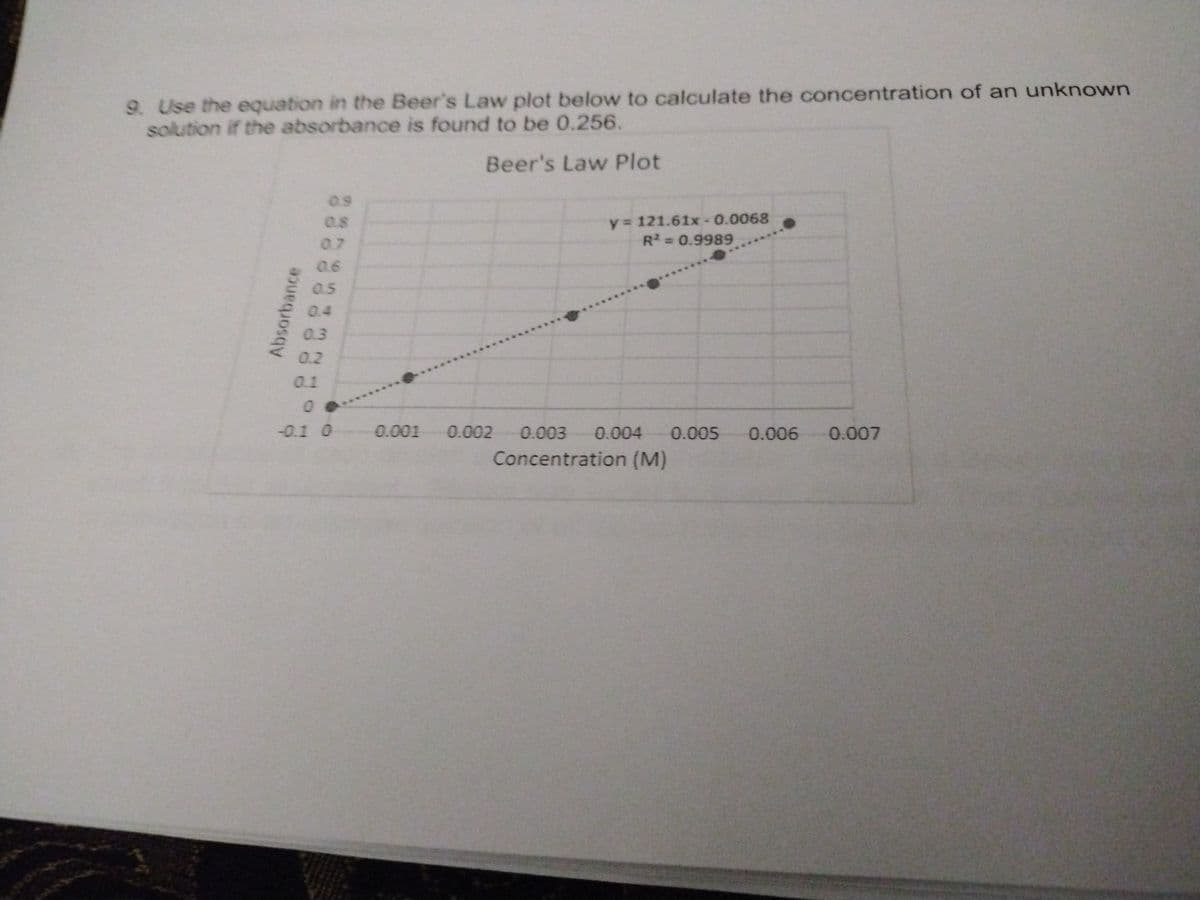
9. Use the equation in the Beer's Law plot below to calculate the concentration of an unknown solution if the absorbance is found to be 0.256. Beer's Law Plot 0.9 y= 121.61x- 0.0068 R= 0.9989 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 00 -0.1 0 0.001 0.002 0.003 0.004 0.005 0.006 0.007 Concentration (M) Absorbance
Q: Given the absorbance spectrum shown below, what wavelength of light would you use for nickel(II)…
A: The principle of absorbance is beer-lambert's law ,which states that the absorbance of light is…
Q: that absorbs at the wavelength of analysis will not obey Beer Lambert’s law. In uv-vis…
A: Following are the true and false statements with correct solutions.
Q: 7. Calculate the speed of light in a sugar solution. The index of refraction for the sugar solution…
A:
Q: A student carries out the equilibrium constant experiment following a similar procedure used in the…
A: The equilibrium constant can be written as the ratio of concentration of products to the…
Q: In the gold nanoparticle experiment, why did the two gold nanoparticle solutions have different…
A: Option (d) The concentration of tea affected the size of the nanoparticles, which affected the…
Q: Solution. Vol. of A solution (mL) [methyl red], M Absorbance 1…
A: concentration of methyl red (x axis) ) in terms…
Q: In Beer's Law, when the absorbance of a solution is 0, this means that a. all of the incident…
A: The relationship between intensities and absorbance is given as shown below. A=logI0I Here, A is the…
Q: Blue dye can be decolorized by treatment with bleach. The kinetics of the reaction can be studied by…
A:
Q: A relationship between concentration in mg/dL and light absorption in absorbance units is given by…
A:
Q: Take a look at this graph. The reactant curve is plotted in red, while the product cur plotted in…
A: A question based on quantitative analysis, which is to be accomplished.
Q: Consider the reaction from the previous question Fe) +SCN(g) →→→ FeSCN2 (aq) Initially [Fe³+] =…
A:
Q: 31. If the % transmittance of a solution is measured to be 35.2%, what is its absorbance? A) 0.352…
A:
Q: a) A current path shaped as shown in Figure produces a magnetic field at P, the center of the arc.…
A: Given, Current in the wire, I =2 A Angle of the arc, Ө = 30o Radius of the arc, R = 0.4 m Magnetic…
Q: 2. Concentration of metal ion. Absorbance of metal ion in solution 0.316 Concentration of metal ion…
A: 2. Given: Initial Volume of Unknown Sample = 20 mL Volume of Final diluted sample = 25 mL…
Q: Chemistry Select the choice with words that best fills in the blanks in the following statement The…
A:
Q: Briefly explain why it is NOT accurate to calculate the concentration of a sample whose absorbance…
A: The concentration of the sample is determined as shown below.…
Q: The nickel level in a soil sample was determined using AAS. A 3.0 g sample was dissolved in acid and…
A: Given: The calibration curve equation is y = 0.0699x + 0.015 Absorbance of the sample = 0.3 Volume…
Q: In this lab exercise, you will make use of Beer's law (also called Beer-Lambert Law) which is stated…
A: Beer-Lambert law: According to this law, the absorption of radiation by the substance depends on the…
Q: A spectrometer was used to measure the light absorbed by three standard solutions containing…
A:
Q: A sample of colored solution in a 1 cm cuvette has absorbance 1.0 at 360 nm. How much of the…
A: The question is based on the concept of absorbance. we have been given absorbance value. we need to…
Q: Determining the absorbance of known concentrations for Solution A: What is the most sensitive…
A: A question based on tools in analytical chemistry that is to be accomplished.
Q: For A crystal violet hydroxide lab, The procedure requires to set the wavelength at 595 nm. Why do…
A:
Q: The slope for the line is 71.22. Use the Beer's Law plot provided to determine the concentration for…
A: Use Beer's law for the calculation of the concentration of the solution.
Q: A solution was made and its absorbance was measured to be A. If the path length of the light passing…
A: Absorbance is quantity of light absorbed by solution. We use Beers Lambert la to calculate quantity…
Q: In determining the amount of copper and the identity of the copper compound in the unknown sample, a…
A: As 100 mL solution contains 120.8 mg sample so, 5 mL ,, ,, (120.8 mg * 5…
Q: The equation of your standard curve of absorbance versus concentration (mM) is y = 1.245x + 0.0012.…
A: Beer’s Law- The absorbance of solution is directly proportional to the concentration(c) and path…
Q: Absorbance at 580 nm Cunce 1.00 ppm 0.030 2.50 ppm 0.072 5.00 ppm 0.147 7.50 ppm 0.217 10.00 ppm…
A: The solution is given below -
Q: A water sample has been analyzed for phosphate using UVVS method at 662 nm wavelength. The molar…
A: A numerical problem based on Beer-Lambert law, which is to be accomplished.
Q: 10. In what ways can a refractive index be used to characterize a liquid sample? 11. Describe how to…
A: A question based on properties of liquids that is to be accomplished.
Q: This graph shows an absorbance vs concentration curve for Co2"(aq) solution. Sample Standard Curve…
A: The absorbance v/s concentration curve given is,
Q: A Beer's Law graph obtained by plotting absorbance versus concentration in mol/L for a series of…
A:
Q: The observed intensity of light (I) after passing through the sample cell is one-fourth the initial…
A: According to Beer-Lambert’s law :
Q: Look at the questions below and answer true (T), false (F) . 1( ) Intensity of the rays coming and…
A: Hello. Since the question has multiple sub-parts, the first three sub-parts shall only be solved in…
Q: Please complete Table 2 and show the calculations for protein concentration and the concentration of…
A: A question based on tools in analytical chemistry that is to be accomplished.
Q: Differentiate between constructive interference and destructive interference?
A: The phenomenon of interaction of wave with each other is known as interference. Depending upon the…
Q: The concentration and absorbance data below were collected for compound X. A sample of compound X of…
A: Absorbance is related to concentration by the following relation.…
Q: 15. A series of five standard copper solutions are prepared, and the absorbances are measured as…
A: A C(PPM)…
Q: A solution is prepared by diluting 2.79 mL of the blue dye stock solution to 25.00 mL. The measured…
A: Given , volume of blue dye stock solution = 2.79 mL. and the final volume is = 25.00 mL. Blue dye…
Q: The absorbance of an ionic aqueous solution is 0.387. What is the ratio of intensities of the…
A: The absorbance of a solution can be expressed as the negative logarithm of the ratio of incident…
Q: Which of the following variables does absorbance not depend upon? O Conductivity of the sample. O…
A: According to lambert Beer's Law: A = Absornace = molar absorptivity of solute =length of light…
Q: Using the given data compute for the sodium concentration of the patient. Use the same formula in…
A:
Q: Determine the absorbance of a solution that allows 73 units of light through from a light source…
A: The beer’s law explain that the concentration of a solution is directly proportional to the…
Q: To determine the amount of iron thiocyanate in a polluted water sample. We prepared a series of…
A:
Q: You left a glass full of homogenized milk on a table undisturbed for half an hour. What would result…
A: To the eyes, milk may appear homogeneous but it is a heterogeneous mixture. However, homogenized…
Q: The best fit curve line to an absorbance vs. concentration plot for standard solutions of a dye has…
A: Given Slope (m) = 0.1815 ml/mg Intercept (c) = 0.0477 Absorbance ( y-axis) = 0.464
Q: You diluted a concentrated dye by mixing 45 μL of the stock solution with 655 μL of water and…
A: A solution can be diluted in the same container by introducing extra solvent to the existing…
Q: According to Beer’s law, which of the following is true? If the absorbing species is the…
A: According to Beer’s law, the absorbance of species is directly proportional to the concentration of…
Q: Solve the problems using a step-by-step process (solutions). 1. If, with a beam of monochromatic…
A: Formula used :- A = log(1/T) Where A is absorbance and T is transmittance
Q: Given the following sample concentrations and corresponding absorbances, construct a standard curve…
A: We have sample concentration with their absorbance. We know that, Absorbance = molar extinction…
Q: The spectroscopic data in the table is generated with five solutions of known concentration.…
A:
Use the equation in the Beer's Law plot below to calculate the concentration of an unknown solution if the absorbance is found to be 0.256
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

- Solution. Vol. of A solution (mL) [methyl red], M Absorbance 1 10.00 _1.64 x10^-16_ _0.256_ 2 15.00 _2.45 x10^-16_. _0.373_ 3 20.00 _3.27 x10^-16_. _0.486_ 4 25.00 _4.09 x10^-16_. _0.620_ Plot a graph of the absorbance (y axis) as a function of the concentration of methyl red (x axis). What is the slope of the graph?Assume a plot of our Abs versus concentration (in M) is linear and has a slope of 7600. Calculate the concentration for the colored species, if the absorbance was 0.360.Which of the following is true? A dilute solution with ingredient that absorbs at the wavelength of analysis will not obey Beer Lambert’s law. In uv-vis spectroscopy, the higher the absorbance the higher the % transmittance. In uv-vis spectroscopy, shining light to any substance would result in absorbance of the light by the substance regardless of energy of the incident light. Only samples with inherent uv-vis absorbing material can be analyzed by a uv-vis spectrometer.
- Solutions that contain the FeSCN2+ ion are blood red (you will see this at the end of the second quarter of lab). Consider the regions of the visible spectrum in which the compound should absorb light and prepare a rough sketch of absorbance vs. wavelength for each solution. Label λ max on the graph. (Is this question asking for the graph of my data at the end)For A crystal violet hydroxide lab, The procedure requires to set the wavelength at 595 nm. Why do you need to measure the absorbance at this wavelength? Based on this, will the absorbance increase or decrease over time?The concentration of a dilute aspirin solution is 0.000530 M 0.000530 M . Standard solutions of this compound were used to prepare a Beer's law plot which gives a slope of 1550.1 M −1 1550.1 M − 1 . What is the expected absorbance value for the aspirin solution?
- You are measuring the concentration of an unknown protein sample, and the absorbance at 595 nm (or A595) of your unknown is greater than that of the highest-concentration standard solution. It is not advisable to simply extrapolate the line of the standard curve to calculate the concentration of the unknown In this scenario, what would be the best strategy to measure the concentration of your unknown (instead of extrapolating from the standard curve)?Using beer lambert's law: A= e.c.l ( l is the path length=1cm) determine the extinciton coeffiecient from the absorbance vs concentration graph.Why must solutions with high concentrations be diluted prior to analysis via Beer's Law? A. The relationship between absorbance and concentration is not linear at high concentrations. B. The detector will reach its detection threshold. C. The photon source is too weak to provide accurate results. D. There is no need to work with dilute concentrations; any concentrations will work. E. The molar absorptivity of a compound is dependent on its concentration.
- You are measuring the concentration of an unknown protein sample, and the absorbance at 595 nm (or A595) of your unknown is greater than that of the highest-concentration standard solution. It is not advisable to simply extrapolate the line of the standard curve to calculate the concentration of the unknown In this scenario, what would be the best strategy to measure the concentration of your unknown (instead of extrapolating from the standard curve)? (answer in 5 sentences)Compute for the linear equation of the following concentration and absorbance given in the table. y=0.6169x+0.1031 y=0.6469x+0.1231 y=0.7169x+0.2031 y=0.5169x+0.1531A Beer’s law plot is prepared by plotting absorbance vs molar concentration of the absorbing species. What are the units of the slope? What are the units of the y-intercept?

