2 Question Balance the chemical equation. Based on the equation, how many grams of bromine are produced by the complete reaction of 11 grams of potassium bromide? Use the periodic table e to get the weights of the elements. Carry out your calcuation in the same way as you did in parts A, B, and C. That is, begin by converting grams of KBr to moles of KBr, then use the mole ratio of KBr to Br,. Drag the labels to the correct locations to complete the analysis. Each label can be used more than once. Cl2 + KBr - _KCI + Br2
2 Question Balance the chemical equation. Based on the equation, how many grams of bromine are produced by the complete reaction of 11 grams of potassium bromide? Use the periodic table e to get the weights of the elements. Carry out your calcuation in the same way as you did in parts A, B, and C. That is, begin by converting grams of KBr to moles of KBr, then use the mole ratio of KBr to Br,. Drag the labels to the correct locations to complete the analysis. Each label can be used more than once. Cl2 + KBr - _KCI + Br2
Chapter11: Solving Equilibrium Problems For Complex Systems
Section: Chapter Questions
Problem 11.3QAP
Related questions
Question
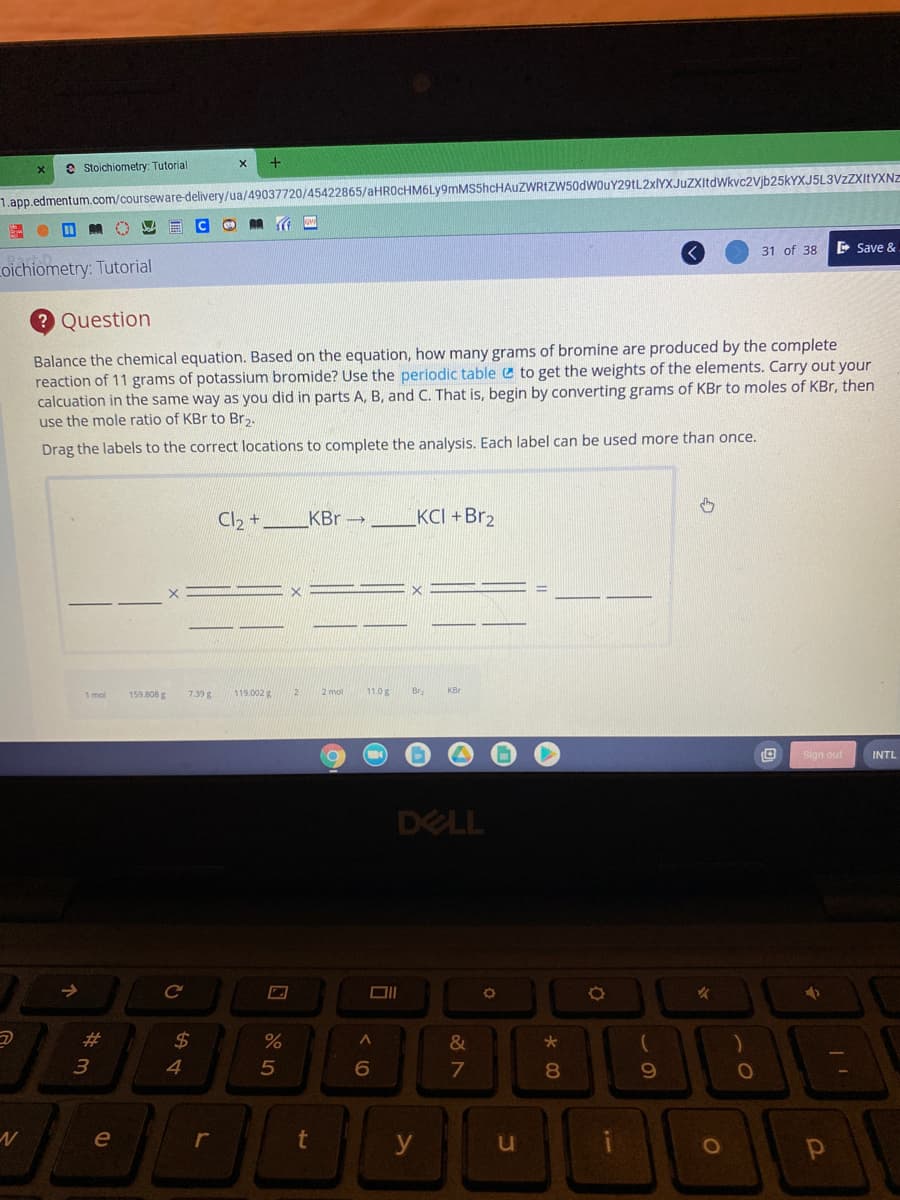
Transcribed Image Text:e Stoichiometry: Tutorial
1.app.edmentum.com/courseware-delivery/ua/49037720/45422865/aHROCHM6Ly9mMS5hcHAuZWRIZW50dwouY29tL2xlYXJuZXitdWkvc2Vjb25kYXJ5L3VzZXItYXNZ
31 of 38
E Save &
oichiometry: Tutorial
Question
Balance the chemical equation. Based on the equation, how many grams of bromine are produced by the complete
reaction of 11 grams of potassium bromide? Use the periodic table e to get the weights of the elements. Carry out your
calcuation in the same way as you did in parts A, B, and C. That is, begin by converting grams of KBr to moles of KBr, then
use the mole ratio of KBr to Br,.
Drag the labels to the correct locations to complete the analysis. Each label can be used more than once.
Cl, +
KBr →
KCI +Br2
1 mol
159.808 g
7.39 g
119.002 g
2 mol
11.0g
Br
KBr
Sign out
INTL
DELL
->
%23
%24
&
4
7
8.
le
r
t
y
1O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning