2. 0.349 g of CaC2•2H2O and 0.698 g of NazCO; are dissolved in 100 mL of water to form a solution. a. What is the limiting reactant? b. How many grams of CaCO; will precipitate? c. How many grams of the excess reactant remain after the reaction has gone to completion?
2. 0.349 g of CaC2•2H2O and 0.698 g of NazCO; are dissolved in 100 mL of water to form a solution. a. What is the limiting reactant? b. How many grams of CaCO; will precipitate? c. How many grams of the excess reactant remain after the reaction has gone to completion?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 62QAP: Ten mL of concentrated H3PO4 (91.7% by mass, d=1.69g/mL) was accidentally poured into a beaker...
Related questions
Question
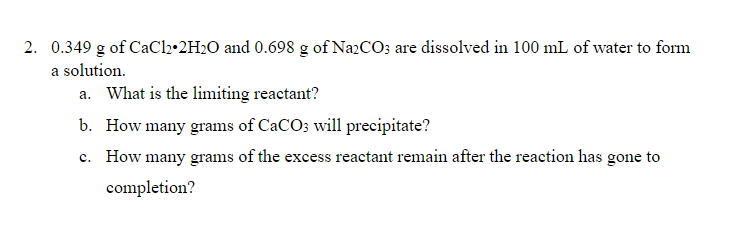
Transcribed Image Text:2. 0.349 g of CaCl2•2H2O and 0.698 g of NazCO; are dissolved in 100 mL of water to form
a solution.
a. What is the limiting reactant?
b. How many grams of CaCO; will precipitate?
c. How many grams of the excess reactant remain after the reaction has gone to
completion?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning