2. 0.4852 g sample of iron ore is dissolved in acid, oxidized to +3 state and precipitated as Fe,03XH,0. The precipitate is filtered, washed and ignited at 1000 °C to Fe,0, (159.6882) which weighed 0.2481 g. Calculate % Fe (55.845) in the sample.
Q: what wt. of limestone containing 9.57% Mg must be taken for analysis in order to precipitate of…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: A 0.7352g sample of ore containing Fe3+, Al3+ and Sr2+ was dissolved and made up to 500.00 mL. The…
A: Given: mass of ore = 0.7352 g Concentration of EDTA = 0.02145 mol/L At pH = 1.0, the volume of EDTA…
Q: The arsenic in a 1.209-g sample of a pesticide was converted to H3 AsO4 by suitable treatment. The…
A: Here we are required to find the percentage of arsenic oxide in sample.
Q: Explain why colour of KMnO4 disappears when oxalic acid is added to its solution in acidic medium.
A: The progress of different acid base reactions can be checked with the help of acid-base indicators.…
Q: A 0.9352g sample of ore containing Fe³+, Al³+ and Sr²+ was dissolved and made up to 500.00 mL. The…
A: A numerical problem based on concentration terms that is to be accomplished.
Q: When treated with a solution of ammonium sulfate an unknown solution produced heavy white…
A: The above procedure is carried out to identify unknown solutions.
Q: The arsenic in a 1.010 g sample of a pesticide was converted to H3AsO4 by suitable treatment. The…
A: step: 1 of 3 We have to calculate the percentage of in 1.010 g pesticide. Reaction of arsenic…
Q: The arsenic in a 1.203-g sample was converted to H,AsO4 by suitable treatment. The acid was then…
A: Given data: Weight of pesticide = 1. 203 g Pesticide →H3AsO4→AgNO3 Ag3AsO4 Ag++SCN- →AgSCN(s)…
Q: Discuss Quantitative aspects of Electrolysi/
A: Electrolysis is a process of decomposition of ionic compounds into their constituent ions on passing…
Q: The arsenic in a 1.010 g sample of a pesticide was converted to H3ASO4 by suitable treatment. The…
A:
Q: While working in a metal processing facility, Letlen had accidentally mixed two metal vats together…
A: Given the total mass of the homogenous alloy sample of Cd and unknown metal taken for titration =…
Q: A solution is made by mixing 500.0 ml. of 0.04794 M Na, HASO, with 500.0 mL of 0.02554 M NaOH.…
A: The given data contains, Volume of Na2HAsO4 =500 mlmolarity of Na2HAsO4=0.04794 MVolume of NaOH…
Q: A sample of soluble salt weighs 1.2 g and contains chloride, bromide and iodide. With AgNO3, a…
A: To Calculate the approximate percentage of chlorine, bromine and iodine in the sample
Q: A complexes of the formula (Co(en),CI]CI prepared by the following procedure Dissolve 40 g of COCL,…
A: It is given that 40 g of COCl2.6H2O is dissolved in 125 mL of water and 16 g of en is dissolved in…
Q: Chrome then curiously asked Senku if it was possible to titrate the pure KHA. H2A2H;O sample through…
A:
Q: A 600.0 mg sample consisting of only CaC2O4and Mg C2O4is heated at 500oC converting the two salts to…
A: Answer: This question is based on the stoichiometric calculation where ratio of the mass of a…
Q: 5.0g of an alloy of copper was dissolved in one litre of dilute sulphuric acid. In 20 ml of this…
A: The reaction is written as 2Cu2++2I-⇌2Cu2++I2 and 2Na2S2O3+I2⇌Na2S4O6+2NaI From the reaction it can…
Q: A solution is made by mixing 500.0 mL of 0.03593 M Na, HASO, with 500.0 mL of 0.03135 M NAOH.…
A:
Q: A 516.7-mg sample containing a mixture of K,SO, and (NH,),SO, was dissolved in water and treated…
A: Weight of sample = 516.7 mg
Q: BH4- + 8Ag+ + 8OH- → H2BO3- + 8Ag(s) + 5H2O The purity of a quantity of KBH4 to be…
A: The question is based on the concept of quantitative estimation of potassium tetrahydro borate…
Q: Step 1 :4.9668 g of Na2SO3 was dissolved in 30ml distilled water by heating. Step 2 : Then 6.0895g…
A: In a reaction, the interaction between the two reactants result in the formation of product. The…
Q: A 2.950 sample containing NH,CI, Rb,CO3 and inert material was dissolved to give 250.0 ml of…
A: Therefore,
Q: The sulfur from 4.00g steel is evolved as dihydrogen sulfide gas and titrated with 1.60 mL of…
A:
Q: What is meant by non-stoichiometric defect? Ionic solids which have anionicvacancies due to metal…
A: Metal excess defect and metal deficiency defect are the two main classes of non-stoichiometric…
Q: Discuss whether the indicator method applied for determination of alkalinity is satisfactory, is…
A:
Q: Chrome then curiously asked Senku if it was possible to titrate the pure KHA-H2A•2H2O sample through…
A: As per the guideline, since you have asked multiple questions, we have solved the first question for…
Q: The arsenic in a 1.010 g sample of a pesticide was converted to H3AsO4 by suitable treatment. The…
A: molarity is defined as the moles of solute upon the volume of sample molarity =molesvolume
Q: The arsenic in a 1.203-g sample of a pesticide was converted to H₃AsO₄ by suitable treatment. The…
A:
Q: To analyze the amount of iron (Fe; Mw = 55.85 g/mol) contained in an ore sample, the sample was…
A: Given in the question, Volume of EDTA = 25 ml Molarity of EDTA = 0.2922 M Total moles of EDTA =…
Q: Ten dietary iron tablets with a total mass of 11.066 g were ground and mixed thoroughly. Then 2.998…
A: Given: 10 dietary tablets Mass of 10 dietary tablets = 11.066 g Now, 2.998 g of powdered tablets…
Q: А, В, С + NH3 1. + HNO3, 2. toluene white precipitate white precipitate yellow toluene layer,…
A: Given scheme is:
Q: The arsenic in a 1.208-g sample of a pesticide was converted to H3 AsO4 by suitable treatment. The…
A:
Q: In the analysis of ammonium ferrous sulphate sample for the content of Fe use was made of a weighed…
A:
Q: Orthophosphate (PO4) in river sediment sample is determined by weighing ammonium phosphomolybdate,…
A: Gravimetric factor (G.F.) is the weight of analyte per unit mass of precipitate. It is the ratio of…
Q: An antihistamine sample, brompheniramine maleate, weighing 5.01234 g was dissolved in alcohol and…
A: Mass of an antihistamine sample, Brompheniramine maleate = 5.01234 g Molarity of AgNO3 = 0.3121 M…
Q: Caustic potash that has been exposed to air is found on analysis to contain 90.00% KOH, 2.38% K2CO3…
A:
Q: An unknown salt X reacts with hot conc. H2SO4 to produce a brown coloured gas which intensifies on…
A: Reaction with conc.H2SO4 to produce a brown gas is a common test for nitrates . The brown gas…
Q: An aqueous ethylene glycol (HOCH, CH, OH, FW = 62.07 g/mol) solution with a mass of 220.9 mg is…
A: Answer: mass percent= 8.15 %
Q: What mass of solid Lanthanum (III) oxalate nonahydrate { La2 ( C2O4 )3 ∙ 9 H2O } can be obtained…
A:
Q: When treated with a solution of ammonium sulfate an unknown solution produced heavy white…
A: The ions present in the unknown solution has to be given,
Q: The Sn in a 0.4352 g mineral specimen was reduced to the +2 state and titrated with 29.77 mL of…
A: Redox titration is the titration used to determine the analyte concentration by carrying out a redox…
Q: A sample of material contains the components NaOH, Na2CO3, NaHCO3 , or possible mixtures of these.…
A: Weight of sample = 1 g normality of acid = 1.038 N
Q: The arsenic in a 1.273-g sample of a pesticide was converted to H3 AsO4 by suitable treatment. The…
A:
Q: Develop two methods to quantify Ag+, firstly using acid-base equilibria and then redox equilibria.…
A: An acid is a substance that can enhance the hydrogen ion (H+) concentration when dissolved in an…
Q: 21. Some 1.0 g steel samples are believed to contain about 2.5% cobalt. Ifa 10% excess of the…
A: Molecular weight of cobalt = 59 Molecular weight of 1-nitroso-2-naphthol = 173
Q: A solution is made by mixing 500.0 mL of 0.02018 M Na, HASO, with 500.0 mL of 0.04078 M NaOH.…
A: Solution: From the given data one thing we have clear that this is the type of acid base reaction.…
kindly provide complete solutions in getting the answer since most answered online were not correct. thank you!
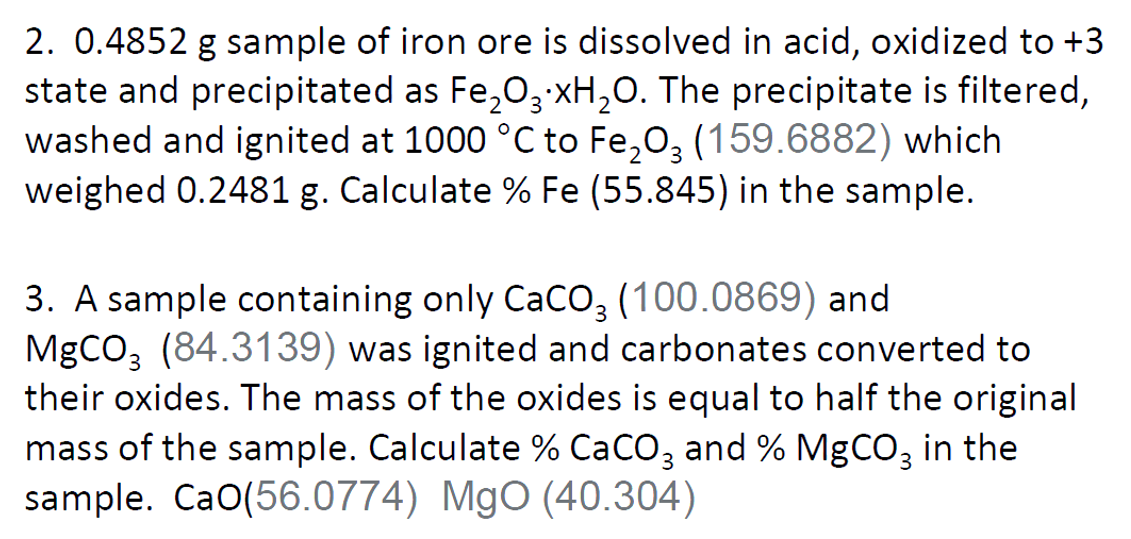
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- What wt of magnetite should be taken for analysis in order that after converting to a precipitate of Fe2O3.xH2O, the percentage of Fe3O4 in the sample can be found by multiplying the wt in grams of the ignited precipitate (Fe2O3) by 100.what wt. of limestone containing 9.57% Mg must be taken for analysis in order to precipitate of 0.551g Mg2P2O7? how many grams of Na2SO4 are required to ppte Ag2SO4 from 2.000t of AgNO3? a sample of magnetite (impure Fe3O4) weighing 0.5000g is fused with oxidizing flux and the ferric compound formed is eventually precipitated as ferric hydroxide and ignited to ferric oxide which weighs 0.4980calculate %Fe & %Fe2O3Calcium fluoride is considered as a relatively insoluble compound and therefore lime or slakedlime has been considered as a possible material to remove excess fluoride in water of boreholesin certain parts of the country. The solubility product of CaF2 is Ksp = 3 x 10 – 11 and that ofCa(OH)2 isKsp =8x10-61. How much lime can be added to the water to remove 10 mg of F- ion per litre ofborehole water?(The atomic masses are Ca: 40.08; F: 19.00; O: 16; H: 1)
- For titration for determination of Chloride, 0.05 AgNO3 and NaCl with a 5% (w/v) K2CrO4 indicator, what is the % (w/w) Cl- using the atomic weight for Chlorine. Data below: Primary standard weight 0.1173g , 0.1056g , and 0.1049g Vol of titrant delivered 35.47mL , 31.75mL, and 31.04mL Uknown Chloride mass 0.1527g, 0.1442g, and 0.1337g Vol of titrant delivered 40.00mL ,37.68mL ,and 34.96 mLThe arsenic in a 1.203-g sample of a pesticide was converted to H3AsO4 by suitable treatment. The acid was then neutralized, and 40.00 mL of 0.05871 M AgNO3 was added to precipitate the arsenic quantitatively as Ag3AsO4. The excess Ag+ in the filtrate and in the washings from the precipitate was titrated with 9.63 mL of 0.1000 M KSCN, and the reaction was. Find the percentage of As2O3 in the sample.A sample containing calcium was analyzed by gravimetry, converting all calcium to calcium oxalate monohydrate (CaC2O4 • H2O). The sample weighing 0.2654 g was dissolved by adding 6M HCl, and then ammonium oxalate solution was added to precipitate all the calcium. The precipitate was suction filtered and dried in an oven at 95 °C for one hour. The solid obtained weighed 0.3216 grams.Calculate:A) The percentage of CaCO3B) The percentage of Ca C) The percentage of CaO
- Prepare 1.0 L of a 50.00 ppm iron solution from the solid Fe(NH4)2(SO4)2 of the80.0% purity. Clearly describe how to prepare this solution. If doneChlorimetric iron analysis using the calibration curve method, specify theform of preparation of 100.0 ml of each of the following standard solutions withcontents of 1.00, 5.00, 15.00 and 20.00 ppm of Fe respectively from the solutionmother (It suppose that the answers are: 0.3180, 2.00, 10.00, 20.00, 30.00 and 40.00 ml of the mother solution and dilute exactly to 100.0 mL with deionized water)A mineral in a fine state of division (0.6324 g) was dissolved in 25.0 mL of 4.0 mol / L boiling HCl and diluted with 175.0 mL of H2O containing two drops of methyl red indicator. The solution was heated to 100 ° C and a heated solution containing 2.00 g of (NH4) 2C2O4 was added slowly to precipitate CaC2O4. Next, NH3 6.0 mol / L was added until the indicator changed from red to yellow, indicating that the liquid was neutral or slightly basic. After slow cooling for 1 hour, the liquid was decanted, the solid transferred to a crucible and washed five times with 0.10 wt% (NH4) 2C2O4 solution, until no Cl- was detected in the filtrate with the addition of AgNO3 solution. The crucible was dried at 105 ° C for 1 hour and then taken to an oven at 500 ° C ± 25 ° C for two hours. The mass of the empty crucible was 18.2311 g. The crucible mass with CaCO3 (s) was weighed 5 times to an average of 18.5467 g. Determine the percentage, by mass, of Ca in the mineral. Ca (40.078 g / mol); C (12.01078…A sample of soluble salts weighs 1.200g and contains chloride, bromide, and iodide. With AgNO3 a ppt is obtained which weighs 0.4500g on heating this ppt in Cl2 gas, the AgBr and AgI are converted to AgCl, and the ppt then weighs 0.3300g. A similar sample, when treated with palladous chloride, ppt only PdI2, and this ppt weighs 0.900g. Find the approximate percentages of chlorine, bromine, and iodine in the original sample.
- A scientist was tasked to extract Fe from an aqueous suspension that contains 106 g of Fe2O3. The following steps detail the transformation of Fe2O3 (aq) to elemental iron: I. Enough sulfur trioxide gas was bubbled to Fe2O3 aqueous suspension to completely yield ferric sulfate. II. Then, 5.00 M nitric acid was added to ferric sulfate yielding an aqueous solution of ferric nitrate. III. Excess magnesium powder was added to the aqueous solution of ferric nitrate precipitating the solid iron ----- a. Write the balanced chemical reaction and the type of chemical reaction for [I], [II], [III]. Do not forget to indicate the states of the reactants and products (s, l, g).(CHOICES: COMBINATION, SINGLE DISPLACEMENT, DOUBLE DISPLACEMENT) b. What is the final mass of iron? Express final answers in 3 significant figures. c. High-concentration HCl is supposed to be added at the last part of the procedure. Briefly state its purpose.The concentration of ammonia in a cleaning product was determined by back titration.Firstly, 10.00 cm3 of the cleaning product was pipetted into a large conical flask,containing 250.00cm3 of 0.50 mol/l HCl to give Solution A.Following a period of reaction and shaking, 50.00cm3 of Solution A was removed anddiluted to 250 cm3 with water in a volumetric flask to give Solution B.20 cm3 samples of Solution B were titrated against 0.05 mol/l Na2CO3 solution, givingan average titre of 12.45 cm3. i) Write equations for the reactions that have taken place.ii) Determine the concentration of NH3 in the original cleaning product in mol/l,g/l, ppm, and % w/v.0.1 g of the mixture of na2so4 and k2so4 is taken and 100 ml of solution is prepared. 10 ml of this prepared solution is placed in a beaker and some distilled water is added. A mass of 15.5 mg is obtained by precipitation with Bacl2 at PH=5, then filtering and bringing to a constant weight at 800 °C. Calculate the percentages of Na2so4 and K2so4 in the mixture accordingly.

