2. A 25.00mL sample of 0.360M NAOH is being titrated with 0.425M HNO3. a.) What is the pH of the 0.360M N2OH solution at the start of the titration (before any HNO3 is added)? Shase -loy (. 360 m) Answer: 0. 3la NooH + HND3 -> thisopolt -2 b.) What is the pH of the solution after 24.0mL HNO3 has been added? O B6om Naoh Answer:
2. A 25.00mL sample of 0.360M NAOH is being titrated with 0.425M HNO3. a.) What is the pH of the 0.360M N2OH solution at the start of the titration (before any HNO3 is added)? Shase -loy (. 360 m) Answer: 0. 3la NooH + HND3 -> thisopolt -2 b.) What is the pH of the solution after 24.0mL HNO3 has been added? O B6om Naoh Answer:
Chapter14: Principles Of Neutralization Titrations
Section: Chapter Questions
Problem 14.38QAP
Related questions
Question
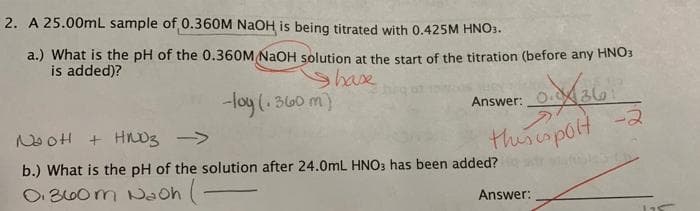
Transcribed Image Text:2. A 25.00mL sample of 0.360M NaOH is being titrated with 0.425M HNO3.
a.) What is the pH of the 0.360M NaOH solution at the start of the titration (before any HNO3
is added)?
Shase
-loy (. 360 m)
Answer:
36
NooH + Hnoz ->
thiscspolt -2
b.) What is the pH of the solution after 24.0mL HNO3 has been added?
O B60m Naoh (-
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning