2. A chemist was charged with the task of making amino alcohol 2. This chemist decided to attempt a Grignard reaction between two equivalents of phenylmagnesium bromide and amino ester 1. To this end, two equivalents of the Grignard reagent were added to a flask containing a stirred THF solution of ester 1 at -78 °C. Upon acidic aqueous work-up of the reaction mixture, the desired alcohol 2 was not isolated, even though one equivalent of methanol was produced and all the starting materials were consumed. Instead, two products with the molecular formulae of C6H6 and C7H₁3NO were obtained in a 2:1 ratio, respectively. Identify the structures of these two products and describe, mechanistically, how they were formed. MgBr + MeO (2 equivalents) O Me Me 1 Me Me HO + MeOH NH2 NH₂ 2
2. A chemist was charged with the task of making amino alcohol 2. This chemist decided to attempt a Grignard reaction between two equivalents of phenylmagnesium bromide and amino ester 1. To this end, two equivalents of the Grignard reagent were added to a flask containing a stirred THF solution of ester 1 at -78 °C. Upon acidic aqueous work-up of the reaction mixture, the desired alcohol 2 was not isolated, even though one equivalent of methanol was produced and all the starting materials were consumed. Instead, two products with the molecular formulae of C6H6 and C7H₁3NO were obtained in a 2:1 ratio, respectively. Identify the structures of these two products and describe, mechanistically, how they were formed. MgBr + MeO (2 equivalents) O Me Me 1 Me Me HO + MeOH NH2 NH₂ 2
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter19: Enolate Anions And Enamines
Section: Chapter Questions
Problem 19.37P
Related questions
Question
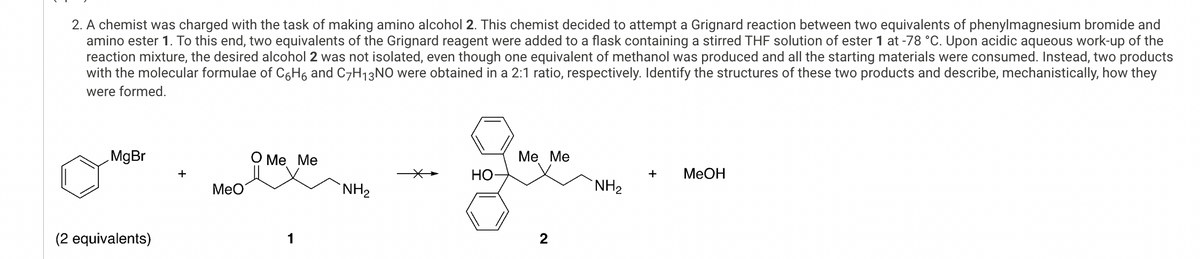
Transcribed Image Text:2. A chemist was charged with the task of making amino alcohol 2. This chemist decided to attempt a Grignard reaction between two equivalents of phenylmagnesium bromide and
amino ester 1. To this end, two equivalents of the Grignard reagent were added to a flask containing a stirred THF solution of ester 1 at -78 °C. Upon acidic aqueous work-up of the
reaction mixture, the desired alcohol 2 was not isolated, even though one equivalent of methanol was produced and all the starting materials were consumed. Instead, two products
with the molecular formulae of C6H6 and C7H₁3NO were obtained in a 2:1 ratio, respectively. Identify the structures of these two products and describe, mechanistically, how they
were formed.
MgBr
+
MeO
(2 equivalents)
O Me Me
1
Me Me
HO
+
MeOH
NH2
NH₂
2
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole