Following is the structural formula of the antihypertensive drug labetalol, a non- specific B-adrenergic blocker with vasodilating activity. Members of this class have received enormous clinical attention because of their effectiveness in treating hypertension (high blood pressure), migraine headaches, glaucoma, ischemic heart disease, and certain cardiac arrhythmias. This retrosynthetic analysis involves dis- connects to the a-haloketone (B) and the amine (C). Each is in turn derived from a simpler, readily available precursor. OH H N. Ph Ph H,N N. H,N НО НО Labetalol (A) CI + H,N. Ph > Ph H,N НО (В) (C) (D) EtO,C H,N Ph НО (E) (F) НО НО PhCH,CI Salicylic acid Benzyl chloride
Following is the structural formula of the antihypertensive drug labetalol, a non- specific B-adrenergic blocker with vasodilating activity. Members of this class have received enormous clinical attention because of their effectiveness in treating hypertension (high blood pressure), migraine headaches, glaucoma, ischemic heart disease, and certain cardiac arrhythmias. This retrosynthetic analysis involves dis- connects to the a-haloketone (B) and the amine (C). Each is in turn derived from a simpler, readily available precursor. OH H N. Ph Ph H,N N. H,N НО НО Labetalol (A) CI + H,N. Ph > Ph H,N НО (В) (C) (D) EtO,C H,N Ph НО (E) (F) НО НО PhCH,CI Salicylic acid Benzyl chloride
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter22: Reactions Of Benzene And Its Derivatives
Section: Chapter Questions
Problem 22.57P
Related questions
Question
Given this retrosynthetic analysis, propose a synthesis for labetalol from salicylic acid and benzyl chloride. [Note: The conversion of salicylic acid to (E) involves a Friedel-Crafts acylation in which the phenolic -OH must be protected by treatment with acetic anhydride to prevent the acylation of the -OH group. The protecting group is later removed by treatment with KOH followed by acidification.]
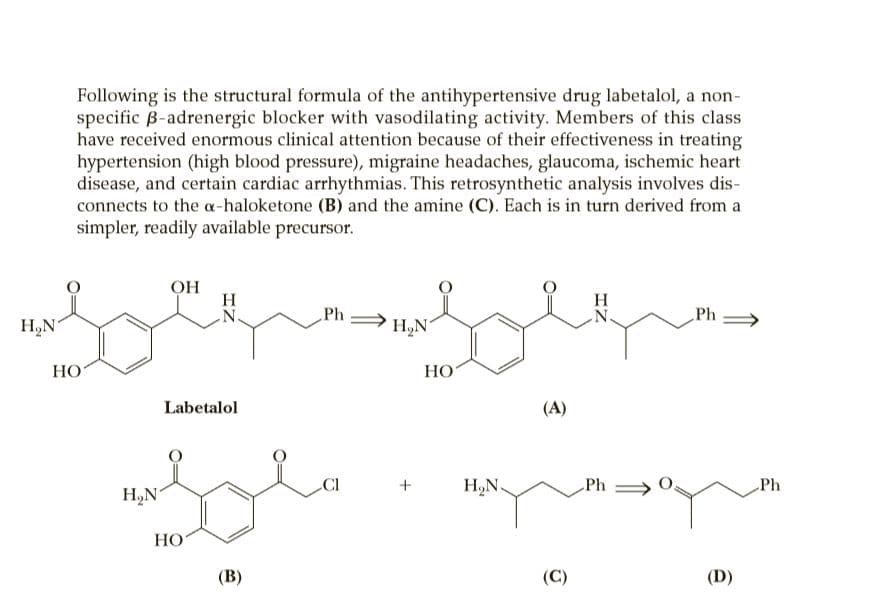
Transcribed Image Text:Following is the structural formula of the antihypertensive drug labetalol, a non-
specific B-adrenergic blocker with vasodilating activity. Members of this class
have received enormous clinical attention because of their effectiveness in treating
hypertension (high blood pressure), migraine headaches, glaucoma, ischemic heart
disease, and certain cardiac arrhythmias. This retrosynthetic analysis involves dis-
connects to the a-haloketone (B) and the amine (C). Each is in turn derived from a
simpler, readily available precursor.
OH
H
N.
Ph
Ph
H,N
N.
H,N
НО
НО
Labetalol
(A)
CI
+
H,N.
Ph >
Ph
H,N
НО
(В)
(C)
(D)
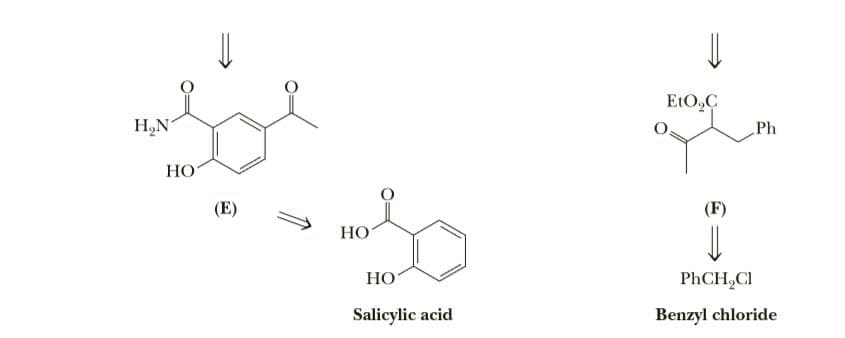
Transcribed Image Text:EtO,C
H,N
Ph
НО
(E)
(F)
НО
НО
PhCH,CI
Salicylic acid
Benzyl chloride
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
