2. (a) In an osmosis cell, one side of its cell was filled with a solution which was made by dissolving 1.04 g of an unknown compound in 175.0 mL of solution at 25 °C. The osmotic pressure of this solution is 1.93 atm. Briefly explain why the osmotic pressure occurred. Determine the concentration of the solution, molar mass and molecular formula of the unknown compound which contains carbon, hydrogen and oxygen if the combustion of 24.02 g of this compound produced 28.16 g CO2 and 8.64 g H2O. (R = 0.0821 L atm mol·' K'', RAM: C = 12.0, H =1.0, O = 16.0)
2. (a) In an osmosis cell, one side of its cell was filled with a solution which was made by dissolving 1.04 g of an unknown compound in 175.0 mL of solution at 25 °C. The osmotic pressure of this solution is 1.93 atm. Briefly explain why the osmotic pressure occurred. Determine the concentration of the solution, molar mass and molecular formula of the unknown compound which contains carbon, hydrogen and oxygen if the combustion of 24.02 g of this compound produced 28.16 g CO2 and 8.64 g H2O. (R = 0.0821 L atm mol·' K'', RAM: C = 12.0, H =1.0, O = 16.0)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 45QAP: Insulin is a hormone responsible for the regulation of glucose levels in the blood. An aqueous...
Related questions
Question
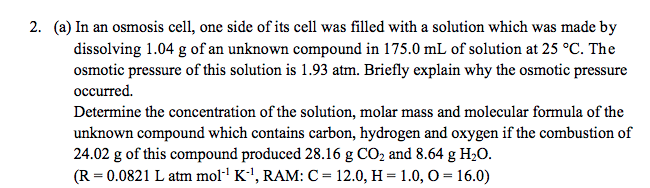
Transcribed Image Text:2. (a) In an osmosis cell, one side of its cell was filled with a solution which was made by
dissolving 1.04 g of an unknown compound in 175.0 mL of solution at 25 °C. The
osmotic pressure of this solution is 1.93 atm. Briefly explain why the osmotic pressure
occurred.
Determine the concentration of the solution, molar mass and molecular formula of the
unknown compound which contains carbon, hydrogen and oxygen if the combustion of
24.02 g of this compound produced 28.16 g CO2 and 8.64 g H2O.
(R = 0.0821 L atm moll K', RAM: C= 12.0, H = 1.0, O = 16.0)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning