2. A molecule with dual polarity has an ionic “head” and a double nonpolar “tail” is often represented by If a sample of this compound (containing lots of molecules) is dropped into a container of benzene (C6H6), draw what you might expect to see if the molecules in the sample have arranged
2. A molecule with dual polarity has an ionic “head” and a double nonpolar “tail” is often represented by If a sample of this compound (containing lots of molecules) is dropped into a container of benzene (C6H6), draw what you might expect to see if the molecules in the sample have arranged
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter7: Molecular Structures
Section: Chapter Questions
Problem 48QRT
Related questions
Question
2. A molecule with dual polarity has an ionic “head” and a double nonpolar “tail” is often represented by
If a sample of this compound (containing lots of molecules) is dropped into a container of benzene (C6H6), draw what you might expect to see if the molecules in the sample have arranged themselves to maximize favorable interactions. Briefly also explain why you drew the arrangement you did.
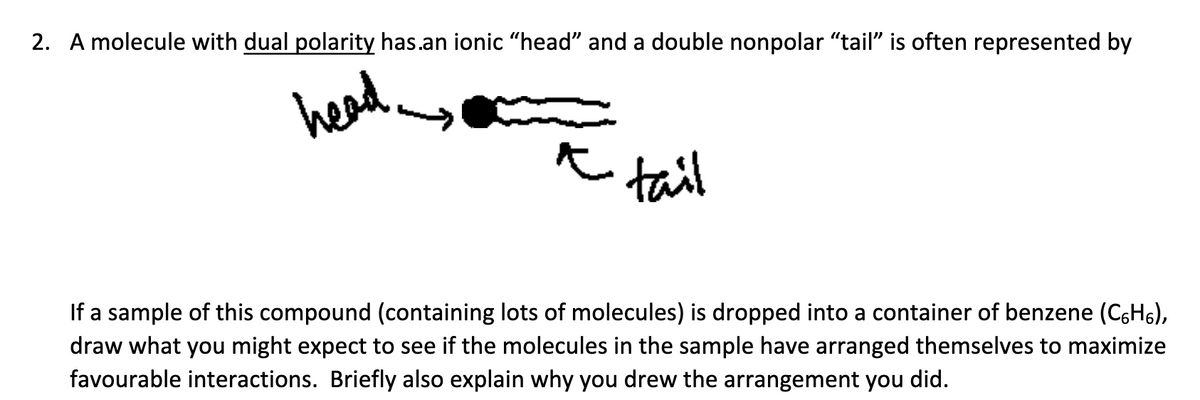
Transcribed Image Text:2. A molecule with dual polarity has.an ionic "head" and a double nonpolar "tail" is often represented by
head
tail
If a sample of this compound (containing lots of molecules) is dropped into a container of benzene (C6H5),
draw what you might expect to see if the molecules in the sample have arranged themselves to maximize
favourable interactions. Briefly also explain why you drew the arrangement you did.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning