2. A reaction was found to proceed mechanistically in two steps. The following data was reported: Step 1: Ext = 35 kl/mol; AHº = +25 kJ/mol Step 2: Enct = 7 kJ/mol; AH° = -45 kJ/mol (a) Draw a potential energy diagram for the complete reaction. Identify the x and y axes, and label the diagram with the following: the transition states; the rate - determining step; the locations of the reactants, intermediates, and products of the reaction; the two Egct values; and the two AH° values given. (b) Calculate the overall AH° for the reaction from the graph, and label your diagram with it. (c) Are the first and second steps endothermic or exothermic? Justify your choices. (d) Are the transition states each for the first and second steps more product - like or more reactant - like? Justify your answers!
2. A reaction was found to proceed mechanistically in two steps. The following data was reported: Step 1: Ext = 35 kl/mol; AHº = +25 kJ/mol Step 2: Enct = 7 kJ/mol; AH° = -45 kJ/mol (a) Draw a potential energy diagram for the complete reaction. Identify the x and y axes, and label the diagram with the following: the transition states; the rate - determining step; the locations of the reactants, intermediates, and products of the reaction; the two Egct values; and the two AH° values given. (b) Calculate the overall AH° for the reaction from the graph, and label your diagram with it. (c) Are the first and second steps endothermic or exothermic? Justify your choices. (d) Are the transition states each for the first and second steps more product - like or more reactant - like? Justify your answers!
Chapter5: Stereochemistry At Tetrahedral Centers
Section5.SE: Something Extra
Problem 58AP: One of the steps in fat metabolism is the hydration of crotonate to yield 3-hydroxybutyrate. This...
Related questions
Question
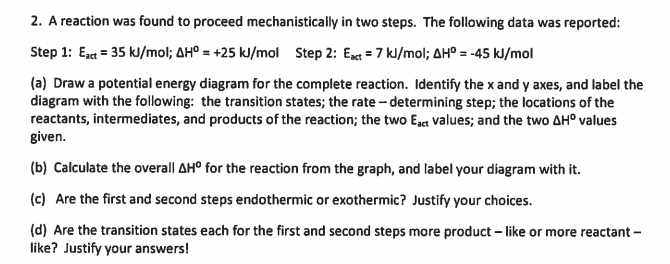
Transcribed Image Text:2. A reaction was found to proceed mechanistically in two steps. The following data was reported:
Step 1: Ez = 35 kJ/mol; AHº = +25 kl/mol Step 2: Eact = 7 kl/mol; AH° = -45 kJ/mol
(a) Draw a potential energy diagram for the complete reaction. Identify the x and y axes, and label the
diagram with the following: the transition states; the rate - determining step; the locations of the
reactants, intermediates, and products of the reaction; the two Ega values; and the two AH° values
given.
(b) Calculate the overall AH° for the reaction from the graph, and label your diagram with it.
(c) Are the first and second steps endothermic or exothermic? Justify your choices.
(d) Are the transition states each for the first
like? Justify your answers!
second steps more product – like or more reactant -
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

