2. A sample of dry ice (solid CO2) is cooled to 173 K (-100.0 °C) and is set on a table at room temperature (298 K; 25 °C). At what temperature is the rate of sublimation and deposition the same (assume that pressure is held constant at 1 atm)?
2. A sample of dry ice (solid CO2) is cooled to 173 K (-100.0 °C) and is set on a table at room temperature (298 K; 25 °C). At what temperature is the rate of sublimation and deposition the same (assume that pressure is held constant at 1 atm)?
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter21: The Solid State: Crystals
Section: Chapter Questions
Problem 21.48E: Which solid phase that is, which allotrope of carbon is more stable, graphite or diamond? You should...
Related questions
Question
answer the number 2
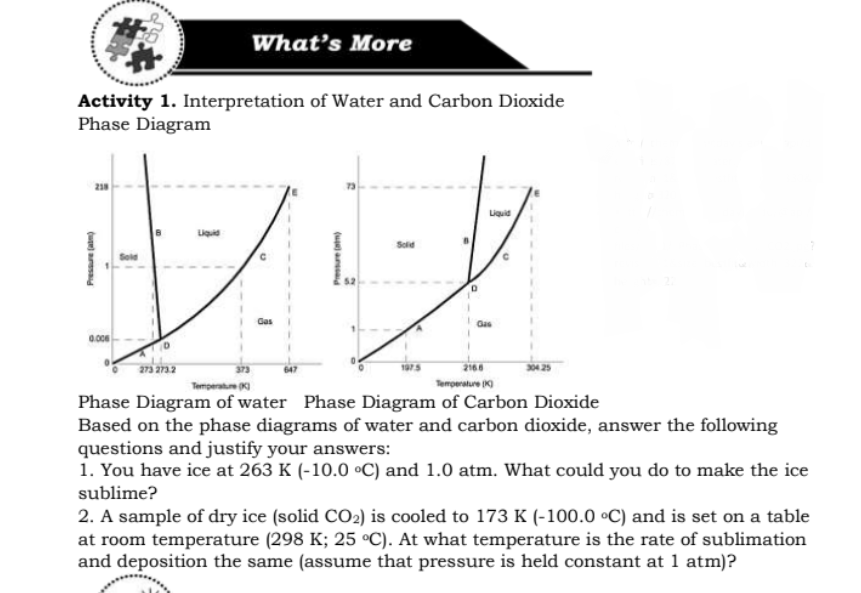
Transcribed Image Text:What's More
Activity 1. Interpretation of Water and Carbon Dioxide
Phase Diagram
Liquid
Liquid
Sold
Sele
273 273.2
373
647
2166
Temperatue
Temperature (
Phase Diagram of water Phase Diagram of Carbon Dioxide
Based on the phase diagrams of water and carbon dioxide, answer the following
questions and justify your answers:
1. You have ice at 263 K (-10.0 °C) and 1.0 atm. What could you do to make the ice
sublime?
2. A sample of dry ice (solid CO2) is cooled to 173 K (-100.0 °C) and is set on a table
at room temperature (298 K; 25 °C). At what temperature is the rate of sublimation
and deposition the same (assume that pressure is held constant at 1 atm)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning