2. a. Referring to the article "Crystal structure of bacterial succinate:quinone oxidoreductase flavoprotein SdhA in complex with its assembly factor SdhE" describe how the chaperone protein SdhE promotes formation of catalytically active succinate:quinone oxidoreductase (SQR) by assisting in the formation of a covalently bound FAD to the SdhA subunit of the enzyme. Refer to figures 6- one paragraph (1) (II) (IV) A C Arg 399 OA HN Arg 399 HN Arg 399 HN O H.O. Arg 399 HN. R287 NH SDHA-H malonate. R390 H* acetate His 45 H CH2 SDHAF2-G78 CH3 N-His 45 CH2 CH3 CH2 Figure 4. The quinone-methide mechanism for autocatalytic covalent flavinylation in complex Il flavoproteins. The amino acid numbering is shown for Escherichia coli SdhA. See details in the text. SdhA, free flavo- protein subunit of E. coli succinate:ubiquinone oxidoreductase. N-His 45 CH3 CH2 His 354 CH3 N-His 45 His 354 B D His 354 His 354 malonate b. Referring to the article, "How an assembly factor enhances covalent FAD attachment to the flavoprotein subunit of complex II" describe the mechanism of attachment of the FAD cofactor. In your answer, discuss how the amino acids shown in figure 4 facilitate covalent attachment of FAD. Identify which polypeptide supplies these amino acids. (one paragraph or bullets) SDHA-R451 FrdA-R390 SdhA-R399 FrdA-R287 OA R451 OA SDHA-R340 H407 SdhA-R286 R340 ---- H296 C. Why is a dicarboxylate ligand needed to initiate flavinylation? (one paragraph referring to figure 7 shown here)
2. a. Referring to the article "Crystal structure of bacterial succinate:quinone oxidoreductase flavoprotein SdhA in complex with its assembly factor SdhE" describe how the chaperone protein SdhE promotes formation of catalytically active succinate:quinone oxidoreductase (SQR) by assisting in the formation of a covalently bound FAD to the SdhA subunit of the enzyme. Refer to figures 6- one paragraph (1) (II) (IV) A C Arg 399 OA HN Arg 399 HN Arg 399 HN O H.O. Arg 399 HN. R287 NH SDHA-H malonate. R390 H* acetate His 45 H CH2 SDHAF2-G78 CH3 N-His 45 CH2 CH3 CH2 Figure 4. The quinone-methide mechanism for autocatalytic covalent flavinylation in complex Il flavoproteins. The amino acid numbering is shown for Escherichia coli SdhA. See details in the text. SdhA, free flavo- protein subunit of E. coli succinate:ubiquinone oxidoreductase. N-His 45 CH3 CH2 His 354 CH3 N-His 45 His 354 B D His 354 His 354 malonate b. Referring to the article, "How an assembly factor enhances covalent FAD attachment to the flavoprotein subunit of complex II" describe the mechanism of attachment of the FAD cofactor. In your answer, discuss how the amino acids shown in figure 4 facilitate covalent attachment of FAD. Identify which polypeptide supplies these amino acids. (one paragraph or bullets) SDHA-R451 FrdA-R390 SdhA-R399 FrdA-R287 OA R451 OA SDHA-R340 H407 SdhA-R286 R340 ---- H296 C. Why is a dicarboxylate ligand needed to initiate flavinylation? (one paragraph referring to figure 7 shown here)
Biology: The Dynamic Science (MindTap Course List)
4th Edition
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Chapter6: Energy, Enzymes, And Biological Reactions
Section: Chapter Questions
Problem 8TYK: Which of the following statements about the allosteric site is true? a. The allosteric site is a...
Related questions
Question
a- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5866609/pdf/pnas.201800195.pdf
b- (Article is no longer available)
Here are the articles provided within the question - They are not needed but are available if additional information is warranted - The figures being mentioned are already provided. This is Biochemoistry, please answer each part to the best of your ability. There are a max of 3 parts due to guidelines and please answer each part with clear and efficient work with answers. Thank you
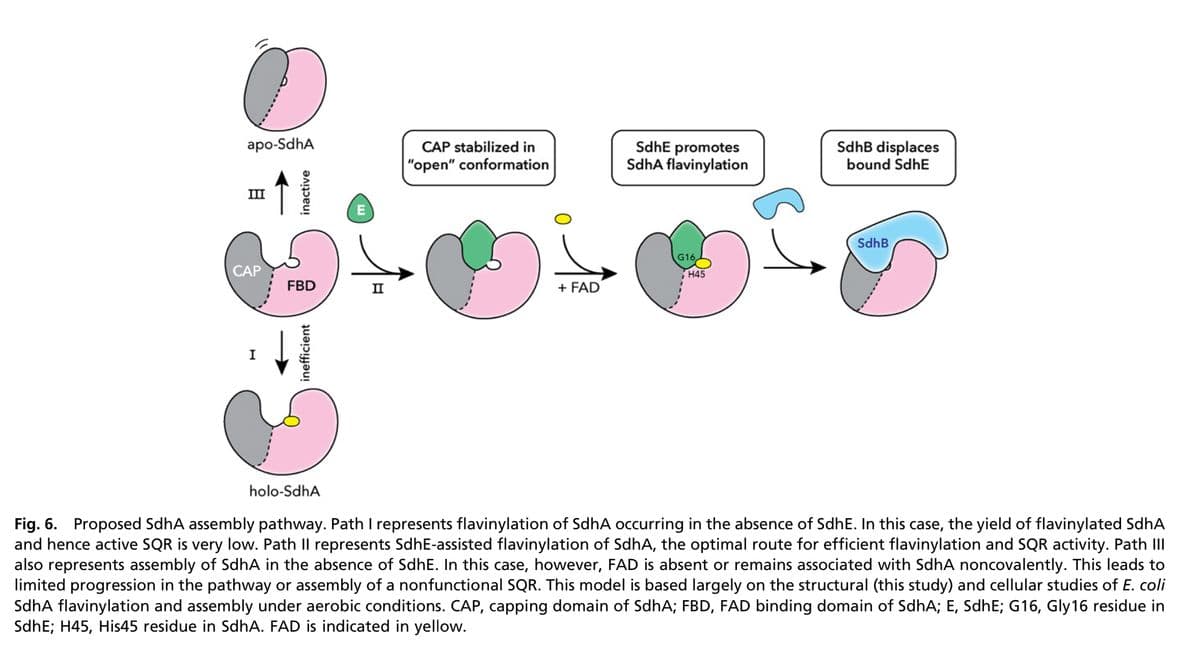
Transcribed Image Text:O
apo-SdhA
III
CAP
I
inactive
FBD
inefficient
E
II
CAP stabilized in
"open" conformation
+ FAD
Sdhe promotes
SdhA flavinylation
G16,
H45
SdhB displaces
bound SdhE
SdhB
holo-SdhA
Fig. 6. Proposed SdhA assembly pathway. Path I represents flavinylation of SdhA occurring in the absence of SdhE. In this case, the yield of flavinylated SdhA
and hence active SQR is very low. Path Il represents SdhE-assisted flavinylation of SdhA, the optimal route for efficient flavinylation and SQR activity. Path III
also represents assembly of SdhA in the absence of SdhE. In this case, however, FAD is absent or remains associated with SdhA noncovalently. This leads to
limited progression in the pathway or assembly of a nonfunctional SQR. This model is based largely on the structural (this study) and cellular studies of E. coli
SdhA flavinylation and assembly under aerobic conditions. CAP, capping domain of SdhA; FBD, FAD binding domain of SdhA; E, SdhE; G16, Gly16 residue in
SdhE; H45, His45 residue in SdhA. FAD is indicated in yellow.
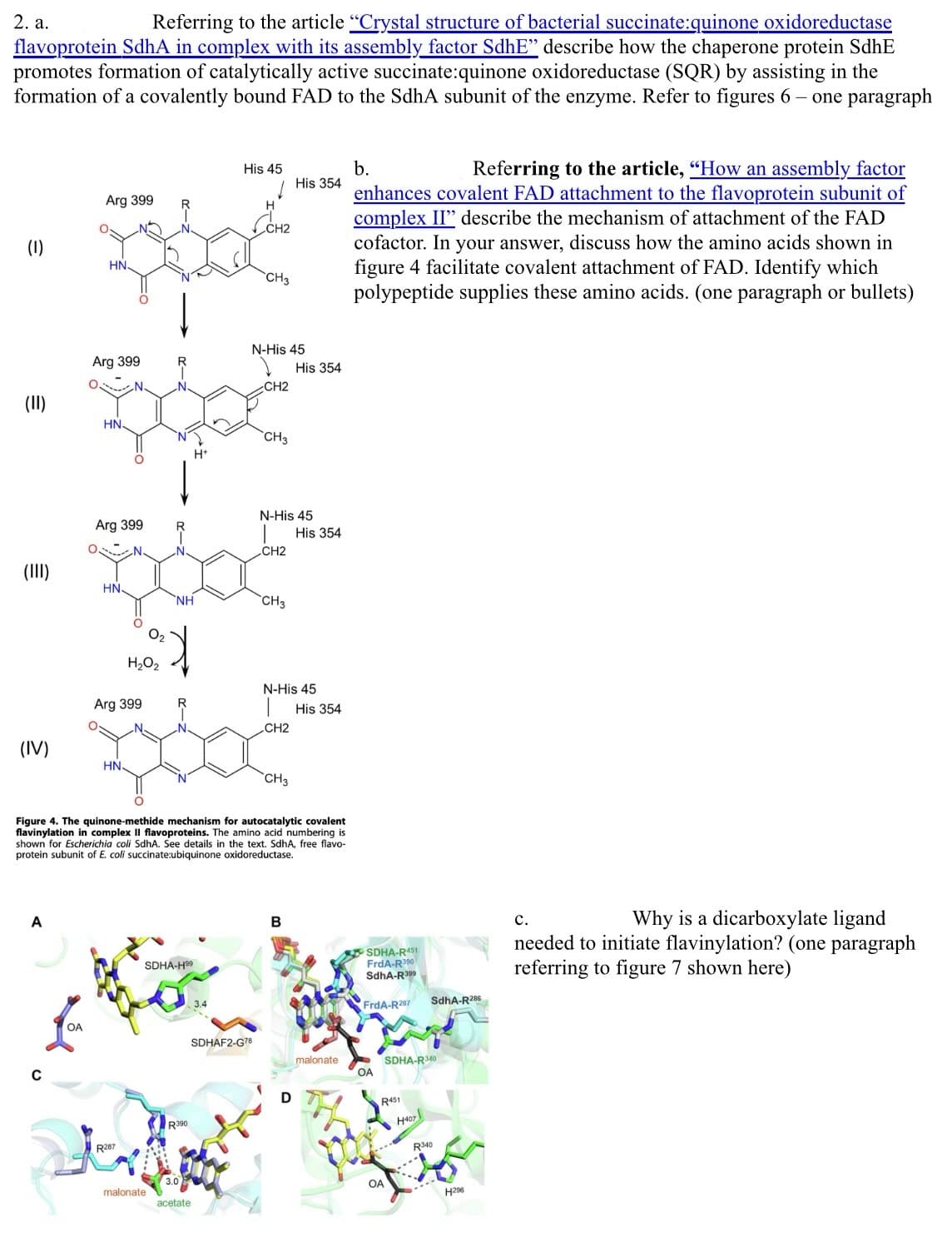
Transcribed Image Text:2. a.
Referring to the article "Crystal structure of bacterial succinate:quinone oxidoreductase
flavoprotein SdhA in complex with its assembly factor SdhE" describe how the chaperone protein SdhE
promotes formation of catalytically active succinate:quinone oxidoreductase (SQR) by assisting in the
formation of a covalently bound FAD to the SdhA subunit of the enzyme. Refer to figures 6- one paragraph
(1)
(IV)
A
с
Arg 399
OA
HN
Arg 399
HN.
Arg 399
HN.
Arg 399
Way
H₂O₂
HN.
R287
NH
SDHA-H9⁹
malonate
R390
His 45
acetate
H
CH2
CH3
SDHAF2-G78
N-His 45
CH2
CH3
CH2
Figure 4. The quinone-methide mechanism for autocatalytic covalent
flavinylation in complex Il flavoproteins. The amino acid numbering is
shown for Escherichia coli SdhA. See details in the text. SdhA, free flavo-
protein subunit of E. coli succinate:ubiquinone oxidoreductase.
N-His 45
CH3
CH2
His 354
CH3
N-His 45
His 354
B
D
His 354
His 354
malonate
b.
Referring to the article, "How an assembly factor
enhances covalent FAD attachment to the flavoprotein subunit of
complex II" describe the mechanism of attachment of the FAD
cofactor. In your answer, discuss how the amino acids shown in
figure 4 facilitate covalent attachment of FAD. Identify which
polypeptide supplies these amino acids. (one paragraph or bullets)
SDHA-R451
FrdA-R390
SdhA-R399
FrdA-R287
OA
R451
OA
SdhA-R286
SDHA-R340
R340
H296
C.
Why is a dicarboxylate ligand
needed to initiate flavinylation? (one paragraph
referring to figure 7 shown here)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Step 1: Subunits of SQR
VIEWStep 2: a. How SdhE promotes activation of SQR by assisting in SdhA flavinylation
VIEWStep 3: b. Mechanism of attachment of FAD cofactor with SdhA and role played by various amino acids
VIEWStep 4: c. Why a dicarboxylate ligand is essential to initiate flavinylation
VIEWSolution
VIEWTrending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning