2. Although the hydrocarbon calicene has so far defied synthesis, its dipole moment has been estimated to 5.6 D. Explain why such a large dipole has been calculated for this hydrocarbon. Structures might be helpful as part of your explanation. calicene
2. Although the hydrocarbon calicene has so far defied synthesis, its dipole moment has been estimated to 5.6 D. Explain why such a large dipole has been calculated for this hydrocarbon. Structures might be helpful as part of your explanation. calicene
Chapter19: Aldehydes And Ketones: Nucleophilic Addition Reactions
Section19.5: Nucleophilic Addition Of H2o: Hydration
Problem 7P
Related questions
Question
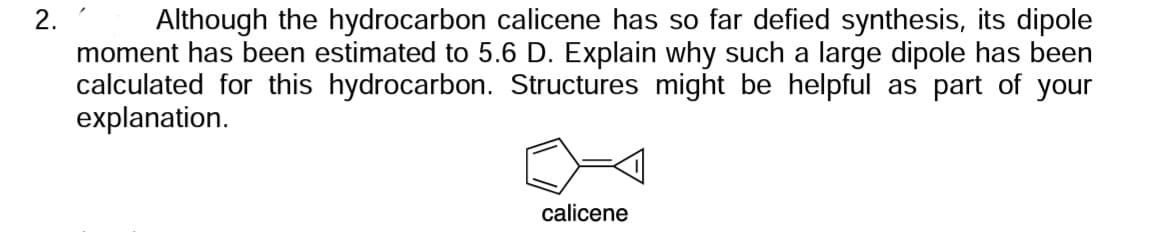
Transcribed Image Text:2.
Although the hydrocarbon calicene has so far defied synthesis, its dipole
moment has been estimated to 5.6 D. Explain why such a large dipole has been
calculated for this hydrocarbon. Structures might be helpful as part of your
explanation.
calicene
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

