2. An aluminum container with specific heat capacity of 0.880 J/g"C contains waxwith specific heat capacity 2.880 J/g°C. The mass of the aluminum container and wax is 160 g while the mass of the container alone is 110 g. How much heat must be added to increase the temperature of the container and wax by 25°C. ( 6020 J)
2. An aluminum container with specific heat capacity of 0.880 J/g"C contains waxwith specific heat capacity 2.880 J/g°C. The mass of the aluminum container and wax is 160 g while the mass of the container alone is 110 g. How much heat must be added to increase the temperature of the container and wax by 25°C. ( 6020 J)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.85PAE: 9.85 The figure below shows a "self-cooling" beverage can. The can is equipped with an outer jacket...
Related questions
Question
Please help me with number 2 only and send paper solutions
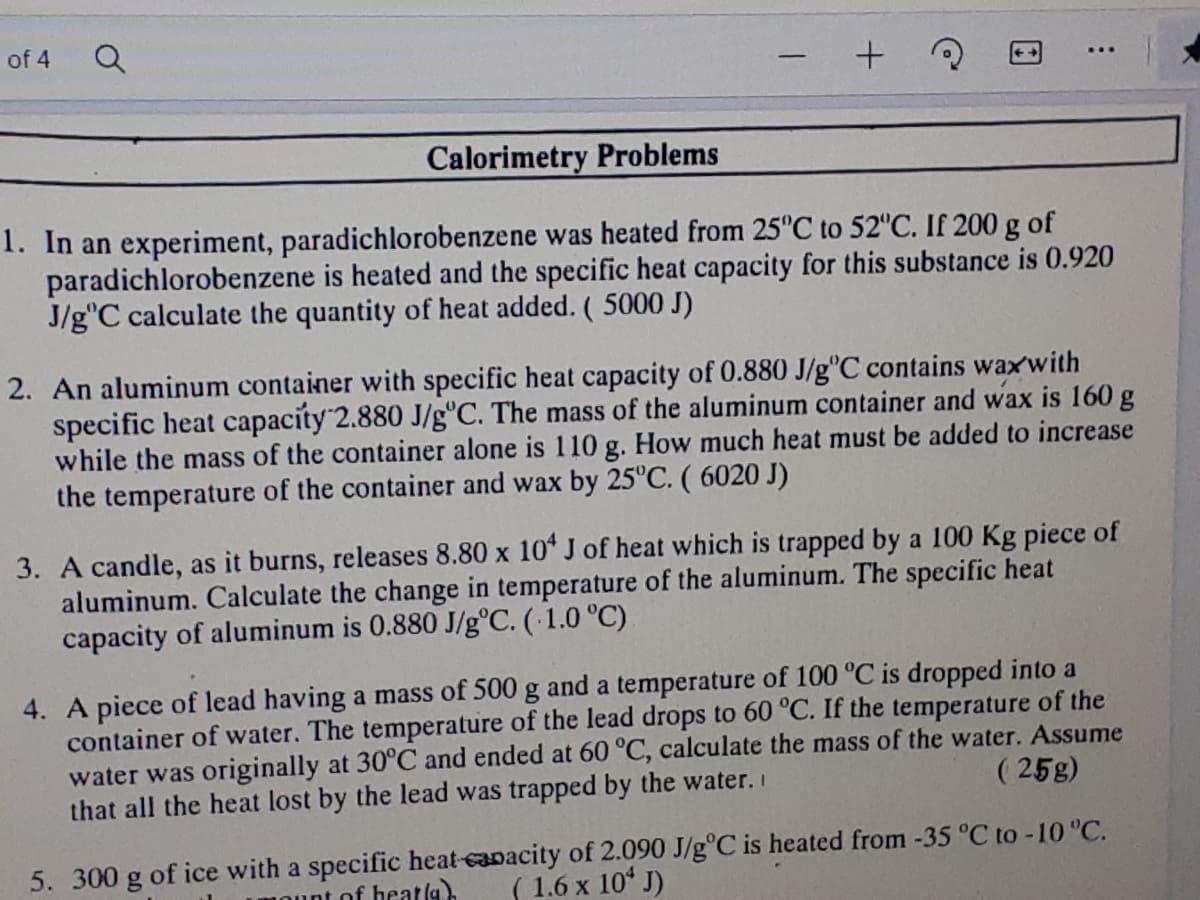
Transcribed Image Text:of 4
Calorimetry Problems
1. In an experiment, paradichlorobenzene was heated from 25°C to 52"C. If 200 g of
paradichlorobenzene is heated and the specific heat capacity for this substance is 0.920
J/g"C calculate the quantity of heat added. (5000 J)
2. An aluminum container with specific heat capacity of 0.880 J/g°C contains waxwith
specific heat capacity 2.880 J/g°C. The mass of the aluminum container and wax is 160 g
while the mass of the container alone is 110 g. How much heat must be added to increase
the temperature of the container and wax by 25°C. ( 6020 J)
3. A candle, as it burns, releases 8.80 x 10* J of heat which is trapped by a 100 Kg piece of
aluminum. Calculate the change in temperature of the aluminum. The specific heat
capacity of aluminum is 0.880 J/g°C. (1.0 °C)
4. A piece of lead having a mass of 500 g and a temperature of 100 °C is dropped into a
container of water. The temperature of the lead drops to 60 °C. If the temperature of the
water was originally at 30°C and ended at 60 °C, calculate the mass of the water. Assume
that all the heat lost by the lead was trapped by the water. I
( 25g)
5. 300 g of ice with a specific heat-capacity of 2.090 J/g°C is heated from -35 °C to -10 "C.
( 1.6 x 10 J)
int of heatla).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning