2. Approximately 1.0 gram of sodium carbonate was added 1. A 100 mL beaker was weighed on a centigram balance. Looking at the photo to the right, record its into the beaker. Looking at the photo to the right, record the mass of the beaker mass to the and sodium carbonate 64.47 nearest 0.01 gram Scout Pro to the nearest 0.01 on your report 62.74 gram (line 2). page (line 1). 3. 8.0 mL of HCl 4. The HCl was poured slowly into the beaker containing the sodium carbonate. The was added into a 10.0 mL graduated cylinder and weighed on a centigram balance. Looking at the photo to the right, record the mass to reaction causes a lot of foaming, so a small amount of acid was added to the beaker and gently swirled until all of the sodium carbonate dissolved. the nearest 0.01 St a gram in your data table (line 4) 12.71 6. The empty graduated cylinder was weighed on a centigram balance. Looking at the photo to the right, record the mass to the nearest 5. When the foaming stopped, the beaker was swirled gently to free any bubbles. 0.01 gram in your data table (line 5) 4.35
2. Approximately 1.0 gram of sodium carbonate was added 1. A 100 mL beaker was weighed on a centigram balance. Looking at the photo to the right, record its into the beaker. Looking at the photo to the right, record the mass of the beaker mass to the and sodium carbonate 64.47 nearest 0.01 gram Scout Pro to the nearest 0.01 on your report 62.74 gram (line 2). page (line 1). 3. 8.0 mL of HCl 4. The HCl was poured slowly into the beaker containing the sodium carbonate. The was added into a 10.0 mL graduated cylinder and weighed on a centigram balance. Looking at the photo to the right, record the mass to reaction causes a lot of foaming, so a small amount of acid was added to the beaker and gently swirled until all of the sodium carbonate dissolved. the nearest 0.01 St a gram in your data table (line 4) 12.71 6. The empty graduated cylinder was weighed on a centigram balance. Looking at the photo to the right, record the mass to the nearest 5. When the foaming stopped, the beaker was swirled gently to free any bubbles. 0.01 gram in your data table (line 5) 4.35
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
Obtain values from the pictures found in the procedure to complete the data table below.
Remember to include units!
A. Data:
10 Mass of beaker + NaCl (after drying)
11 Mass of NaCl Generated
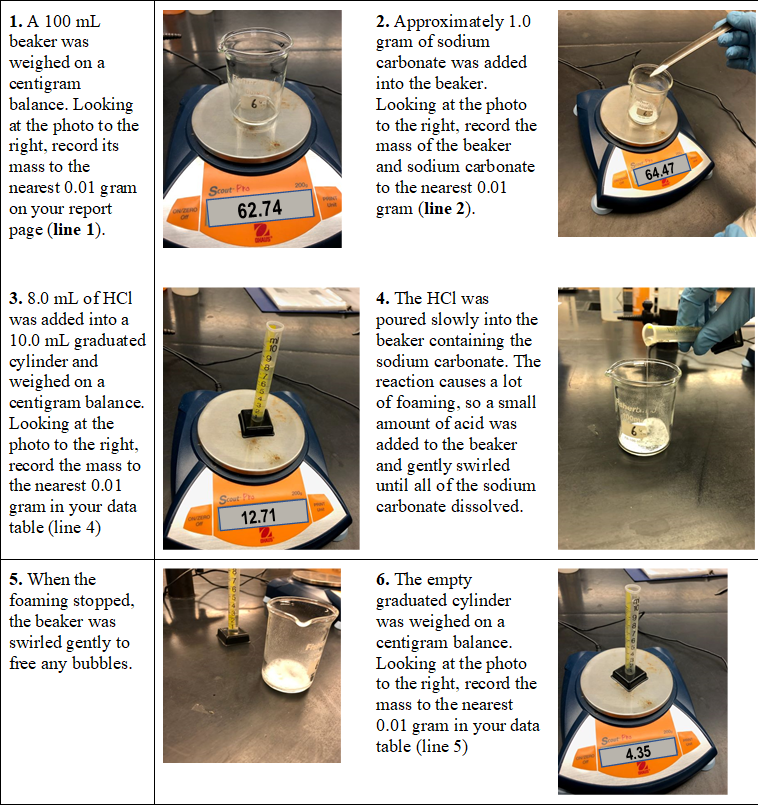
Transcribed Image Text:1. A 100 mL
beaker was
2. Approximately 1.0
gram of sodium
carbonate was added
weighed on a
centigram
balance. Looking
at the photo to the
right, record its
mass to the
nearest 0.01 gram
on your report
page (line 1).
into the beaker.
Looking at the photo
to the right, record the
mass of the beaker
and sodium carbonate
to the nearest 0.01
64.47
Scout Pro
62.74
gram (line 2).
3. 8.0 mL of HCI
4. The HCl was
was added into a
poured slowly into the
beaker containing the
sodium carbonate. The
reaction causes a lot
10.0 mL graduated
cylinder and
weighed on a
centigram balance.
Looking at the
photo to the right,
record the mass to
the nearest 0.01
of foaming, so a small
amount of acid was
added to the beaker
and gently swirled
until all of the sodium
Scout Fa
gram in your data
table (line 4)
carbonate dissolved.
12.71
5. When the
6. The empty
graduated cylinder
was weighed on a
centigram balance.
Looking at the photo
to the right, record the
mass to the nearest
0.01 gram in your data
table (line 5)
foaming stopped,
the beaker was
swirled gently to
free any bubbles.
St
4.35
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
