RADIOACTIVITY. LAW OF RADIOACTIVE DECAY Activity units: 1Ci= 3.7 x 1010 Bq 1. Nuclear decay law: N = Noe t, The law of radioactive decay gives the quantitative relationship between the original number of nuclei present at time zero Ng and the number N at a later time t (sec), where e = 2.71828. is the base of the natural logarithm, and à is the decay constant for the nuclide (1/s). 2. Relationship between decay constant å and isotop half-life T1/2: In(2) - 0.693 Radioisotope Half-life Modality Modality T1/2 T1/a Carton-11 20 minutes PET where A is decay constant (1/s), T1/2 is isotope half- life (s). Fuorine-18 110 minutes PET Copper-64 12.7 hours PET 3. Activity or decay rate of a radioactive source A: Gallum-67 78.3 hours SPECT ΔΝ Galium-68 68 minutes PET = AN Technetum-99m 6.02 hours SPECT Δε where A is activity or decay rate of a radioactive source (Bq), N is number of nuclei present at timet Thalum-201 (s ), a is decay constant (1/s). 4. Activity A of a radioactive source at a time t: 67.3 hours 13.22 hours SPECT Indium-111 SPECT lodine-123 72.9 hours SPECT A = Age dt = A,2 T/a A, is initial activity (Bq), T/2 half life time of an isotope (s). 5. Relationship between mass and number of nuclei: т_ N м м NA m is mass (kg), M is molar mass (kg/mole), N is number of nuclei, NA = 6.022 - 1023 mole is Avogadro's constant. 2. Calculate the activity due to "C in 1.00 kg of carbon found in a living organism. Express the activity in units of Bq and Ci. It is known, that the half-life of "C is 5730 years.
RADIOACTIVITY. LAW OF RADIOACTIVE DECAY Activity units: 1Ci= 3.7 x 1010 Bq 1. Nuclear decay law: N = Noe t, The law of radioactive decay gives the quantitative relationship between the original number of nuclei present at time zero Ng and the number N at a later time t (sec), where e = 2.71828. is the base of the natural logarithm, and à is the decay constant for the nuclide (1/s). 2. Relationship between decay constant å and isotop half-life T1/2: In(2) - 0.693 Radioisotope Half-life Modality Modality T1/2 T1/a Carton-11 20 minutes PET where A is decay constant (1/s), T1/2 is isotope half- life (s). Fuorine-18 110 minutes PET Copper-64 12.7 hours PET 3. Activity or decay rate of a radioactive source A: Gallum-67 78.3 hours SPECT ΔΝ Galium-68 68 minutes PET = AN Technetum-99m 6.02 hours SPECT Δε where A is activity or decay rate of a radioactive source (Bq), N is number of nuclei present at timet Thalum-201 (s ), a is decay constant (1/s). 4. Activity A of a radioactive source at a time t: 67.3 hours 13.22 hours SPECT Indium-111 SPECT lodine-123 72.9 hours SPECT A = Age dt = A,2 T/a A, is initial activity (Bq), T/2 half life time of an isotope (s). 5. Relationship between mass and number of nuclei: т_ N м м NA m is mass (kg), M is molar mass (kg/mole), N is number of nuclei, NA = 6.022 - 1023 mole is Avogadro's constant. 2. Calculate the activity due to "C in 1.00 kg of carbon found in a living organism. Express the activity in units of Bq and Ci. It is known, that the half-life of "C is 5730 years.
University Physics Volume 3
17th Edition
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:William Moebs, Jeff Sanny
Chapter10: Nuclear Physics
Section: Chapter Questions
Problem 6CQ: How is the initial activity rate of a radioactive substance related to its half-life?
Related questions
Question
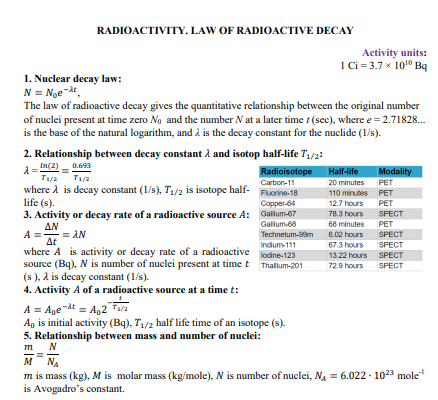
Transcribed Image Text:RADIOACTIVITY. LAW OF RADIOACTIVE DECAY
Activity units:
1Ci= 3.7 x 1010 Bq
1. Nuclear decay law:
N = Noe t,
The law of radioactive decay gives the quantitative relationship between the original number
of nuclei present at time zero Ng and the number N at a later time t (sec), where e = 2.71828.
is the base of the natural logarithm, and à is the decay constant for the nuclide (1/s).
2. Relationship between decay constant å and isotop half-life T1/2:
In(2) - 0.693
Radioisotope Half-life
Modality
Modality
T1/2 T1/a
Carton-11
20 minutes
PET
where A is decay constant (1/s), T1/2 is isotope half-
life (s).
Fuorine-18
110 minutes
PET
Copper-64
12.7 hours
PET
3. Activity or decay rate of a radioactive source A: Gallum-67
78.3 hours SPECT
ΔΝ
Galium-68
68 minutes
PET
= AN
Technetum-99m
6.02 hours
SPECT
Δε
where A is activity or decay rate of a radioactive
source (Bq), N is number of nuclei present at timet Thalum-201
(s ), a is decay constant (1/s).
4. Activity A of a radioactive source at a time t:
67.3 hours
13.22 hours SPECT
Indium-111
SPECT
lodine-123
72.9 hours
SPECT
A = Age dt = A,2 T/a
A, is initial activity (Bq), T/2 half life time of an isotope (s).
5. Relationship between mass and number of nuclei:
т_ N
м м
NA
m is mass (kg), M is molar mass (kg/mole), N is number of nuclei, NA = 6.022 - 1023 mole
is Avogadro's constant.

Transcribed Image Text:2. Calculate the activity due to "C in 1.00 kg of carbon found in a living organism. Express
the activity in units of Bq and Ci. It is known, that the half-life of "C is 5730 years.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning