2. CаН100 100 60 48- 20 3500 2500 1520 1000 6H 1H 1H 2H allle 4.0 3.5 3.0 2.5 1.5 1.0 FPM 0.5 Degree of Unsaturation: Proposed Structure: B0 70 60 ppm 6CCE
2. CаН100 100 60 48- 20 3500 2500 1520 1000 6H 1H 1H 2H allle 4.0 3.5 3.0 2.5 1.5 1.0 FPM 0.5 Degree of Unsaturation: Proposed Structure: B0 70 60 ppm 6CCE
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter7: Cycloalkanes
Section: Chapter Questions
Problem 8CTQ
Related questions
Question
100%
Please help me with these
Transcribed Image Text:2. C4H100
100
80
80-
48-
20
3500
3000
2500
200a
1520
1808
6H
1H
1H
2H
4.0
3.5
3.0
2.5
2.0
1.5
1.0 PPM
0.5
Degree of Unsaturation:
Proposed Structure:
60
50
ppm
6ECE

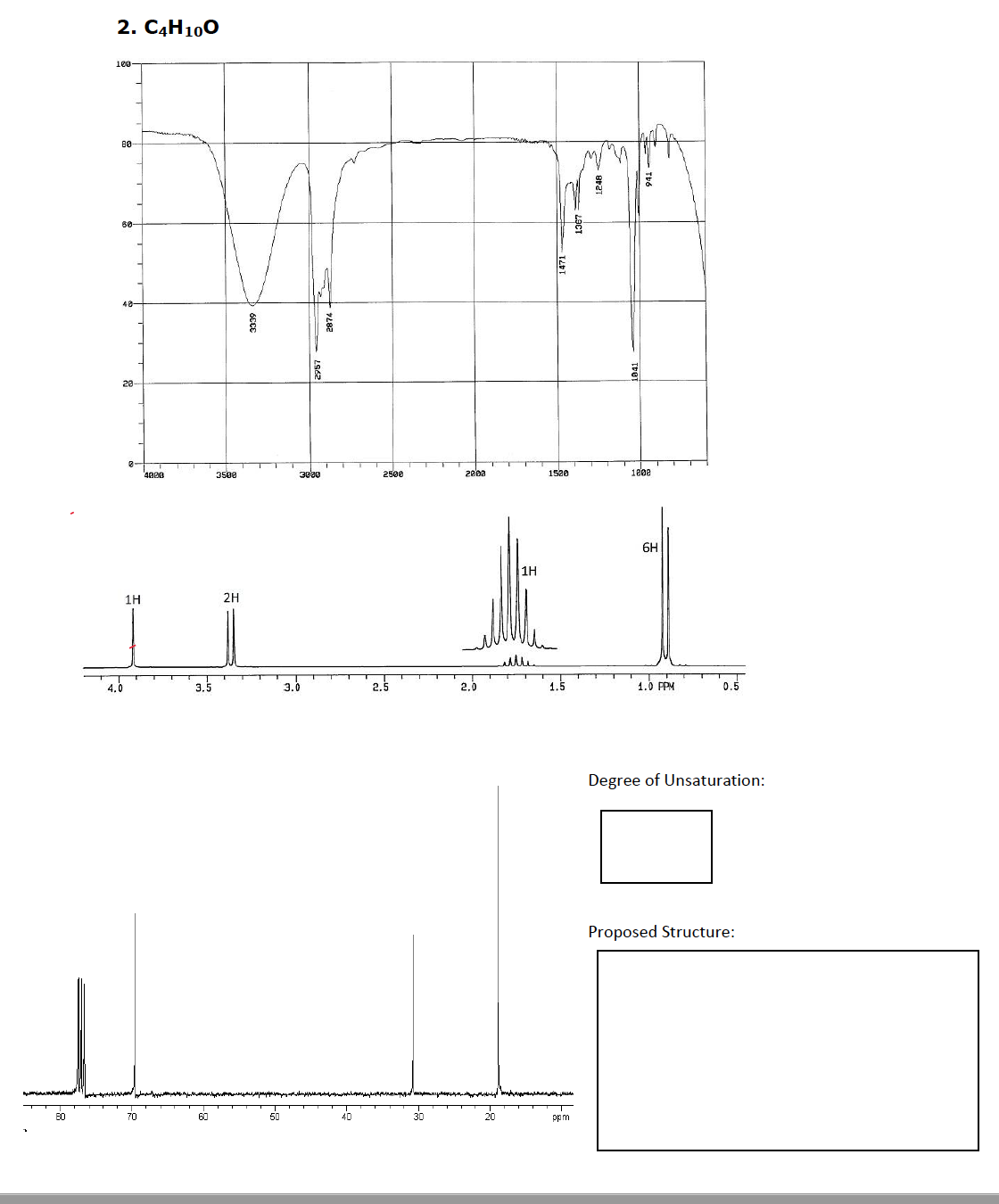
Transcribed Image Text:SPECTROSCOPY DRY LAB
General Formula to calculate the degree of unsaturation:
#C atoms - (#H atoms/2) + 1
Example: Benzene (C6H6) 6 - (6/2) + 1 = 4
Rules for calculating unsaturation with organic compounds that contain heteroatoms
(halogen, O, S, N)
Rule 1) Replace any halogens with hydrogens
Example: CH3B change to CH4 and then calculate 1- (4/2) + 1 = 0
Rule 2) Ignore any O or S atoms in the compound
Example: Phenol (C6H5OH) calculate for C6H6 6- (6/2) + 1 = 4
Rule 3) Subtract a H for every Nitrogen present
Example: Aniline (C6H5NH2) calculate for C6H6 6- (6/2) + 1 = 4
For the following problems given spectral data and the chemical formula:
1) Determine the degree of unsatuaration using the unsaturation rules above and list it in the
appropriate box:
2) Label important absorption bands directly on the IR spectra
3) Identify and label all signals directly on the 1H NMR and or 13C NMR spectra (make sure to
use Ha, Hb, Ca, Cb, etc...
Remember TMS shows up as a singlet at 0 ppm for both the 1H and the 13C NMR
CDCI3 (solvent) sometimes shows up as a triplet around 77pm in a 1°C NMR
4) Propose a structure for the compound that matches 1-3 above (If your structure has more
than four degrees of unsaturation it's quite likely to have an aromatic ring). Draw the
SKELETAL structure in the proposed structure box.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning