2. If ion-selective electrodes for the determination of H*, Na*, Ca2+, S²- have Nernstian responses then a 10-fold increase in the concentration of target ions will increase the potential of the Electrode. for the H* electrode. a) by b) by _for the Ca2+ electrode. 3. The possible electron transitions that occur in a molecule when interact with light with wavelength in the a) UV/Vis range are between different b) IR ranges are between different 4. For photometric measurements in the UV range we use a lamp.
2. If ion-selective electrodes for the determination of H*, Na*, Ca2+, S²- have Nernstian responses then a 10-fold increase in the concentration of target ions will increase the potential of the Electrode. for the H* electrode. a) by b) by _for the Ca2+ electrode. 3. The possible electron transitions that occur in a molecule when interact with light with wavelength in the a) UV/Vis range are between different b) IR ranges are between different 4. For photometric measurements in the UV range we use a lamp.
Chapter10: Potentiometry And Redox Titrations
Section: Chapter Questions
Problem 2P
Related questions
Question
plz solve it within 30-40 mins I'll give you multiple upvote
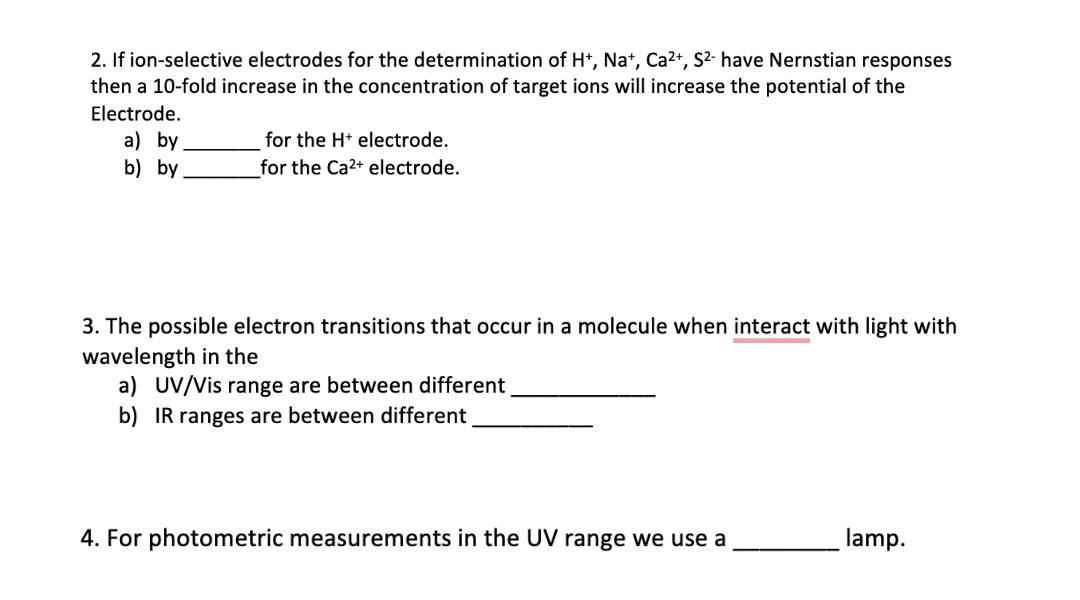
Transcribed Image Text:2. If ion-selective electrodes for the determination of H*, Na*, Ca2+, S²- have Nernstian responses
then a 10-fold increase in the concentration of target ions will increase the potential of the
Electrode.
a) by
b) by
for the H* electrode.
for the Ca2+ electrode.
3. The possible electron transitions that occur in a molecule when interact with light with
wavelength in the
a) UV/Vis range are between different
b) IR ranges are between different
4. For photometric measurements in the UV range we use a
lamp.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
