2. Plot 1/λ (y-axis) vs 1/n2 (x-axis) for hydrogen and attach your graph to the back of this handout. Excel graphs are not permitted. Your graph should have: a title, proper axis scaling, axes labelled including units, data points shown clearly eg. dot in a circle, best straight line drawn, and the coordinates (x,y) of the points on the straight line chosen to calculate the slope. Never use data points to calculate the slope! Show slope calculation directly on the graph. 3. a) From your graph, calculate the experimental value of R, the Rydberg constant. (Note- this is the absolute value of your slope): b) Based on the theoretical value of R, 1.0974 x 107 m1, calculate your % error:
2. Plot 1/λ (y-axis) vs 1/n2 (x-axis) for hydrogen and attach your graph to the back of this handout. Excel graphs are not permitted. Your graph should have: a title, proper axis scaling, axes labelled including units, data points shown clearly eg. dot in a circle, best straight line drawn, and the coordinates (x,y) of the points on the straight line chosen to calculate the slope. Never use data points to calculate the slope! Show slope calculation directly on the graph. 3. a) From your graph, calculate the experimental value of R, the Rydberg constant. (Note- this is the absolute value of your slope): b) Based on the theoretical value of R, 1.0974 x 107 m1, calculate your % error:
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 73QAP: A Different civilization on a distant planet has developed a new temperature scale based on ethyl...
Related questions
Question
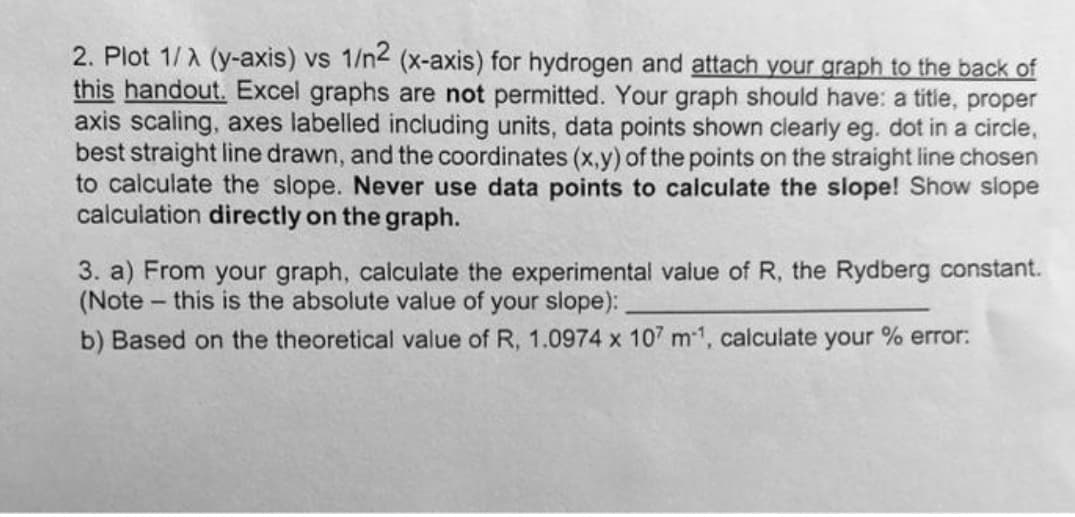
Transcribed Image Text:2. Plot 1/A (y-axis) vs 1/n2 (x-axis) for hydrogen and attach your graph to the back of
this handout. Excel graphs are not permitted. Your graph should have: a title, proper
axis scaling, axes labelled including units, data points shown clearly eg. dot in a circle,
best straight line drawn, and the coordinates (x,y) of the points on the straight line chosen
to calculate the slope. Never use data points to calculate the slope! Show slope
calculation directly on the graph.
3. a) From your graph, calculate the experimental value of R, the Rydberg constant.
(Note- this is the absolute value of your slope):
b) Based on the theoretical value of R, 1.0974 x 107 m1, calculate your % error:

Transcribed Image Text:Line wavelength X(nm) [1/^ (mt)
-น
2.43 X10¹2
12.30 X10-¹7
2.04x60
1.54 x10-12
410.20nm
434.60nm
489.70nm
вид. лопт
-12
ni (calculated) ni (integer)
0.61
0.6
0.5
0.58
0.53
0.44
0.5
0.4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co