2. Suppose a piece of metal at 90 °C is quickly transferred into a Styrofoam cup containing 212 grams of water at 24 °C. After a minute or so, the temperature of the contents of the cup is stable at 35 °C. Use 4.2 J/(g.°C) as the specific heat capacity of water and assume that during this time the energy transferred between the contents of the cup and the surroundings is negligible and that there is no heat loss to the cup. (a) How much heat in joules is gained by the water? (b) How much heat in joules is lost by the metal? (c) What is the heat capacity of this piece of metal? (d) If the mass of the metal is 196 grams, what is its specific heat capacity? (e) What is the change of water's entropy? Note the volume of water stays the same. (f) What is the change of total entropy of the system, including both water and metal?
2. Suppose a piece of metal at 90 °C is quickly transferred into a Styrofoam cup containing 212 grams of water at 24 °C. After a minute or so, the temperature of the contents of the cup is stable at 35 °C. Use 4.2 J/(g.°C) as the specific heat capacity of water and assume that during this time the energy transferred between the contents of the cup and the surroundings is negligible and that there is no heat loss to the cup. (a) How much heat in joules is gained by the water? (b) How much heat in joules is lost by the metal? (c) What is the heat capacity of this piece of metal? (d) If the mass of the metal is 196 grams, what is its specific heat capacity? (e) What is the change of water's entropy? Note the volume of water stays the same. (f) What is the change of total entropy of the system, including both water and metal?
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter2: Matter And Energy
Section: Chapter Questions
Problem 29RQ: If 3000 ft3 of air is crossing an evaporator coil and iscooled from 75F to 55F, what would be the...
Related questions
Question
100%
I need help with parts d, e, and f please.
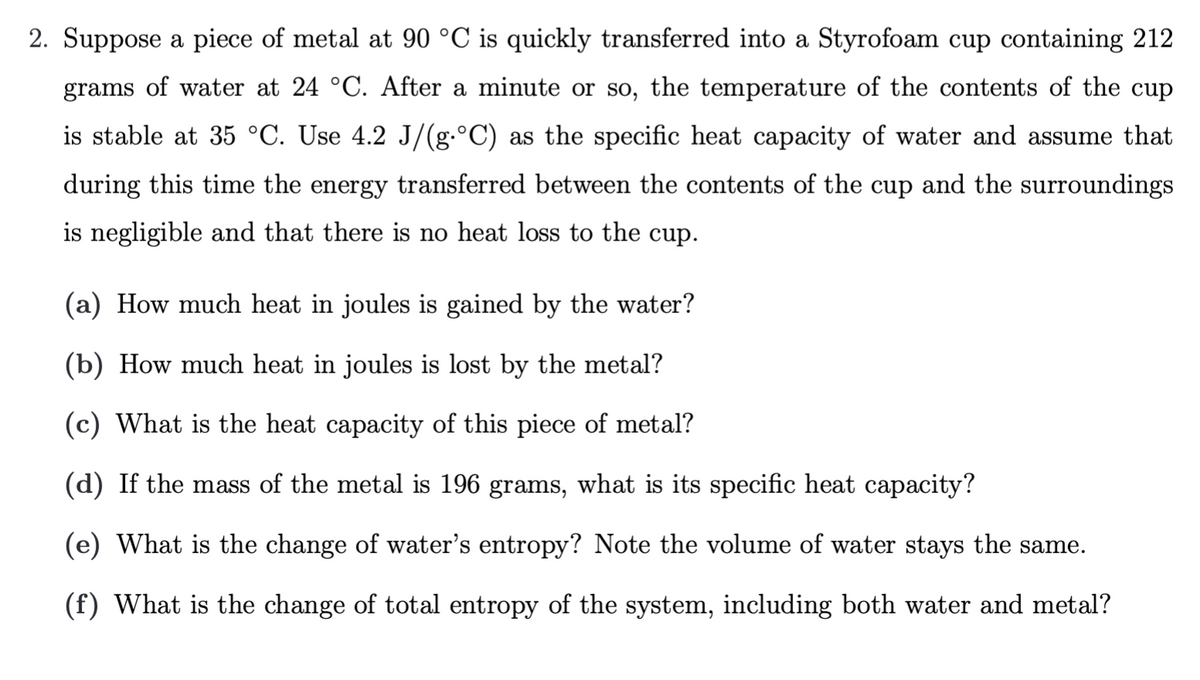
Transcribed Image Text:2. Suppose a piece of metal at 90 °C is quickly transferred into a Styrofoam cup containing 212
grams of water at 24 °C. After a minute or so, the temperature of the contents of the cup
is stable at 35 °C. Use 4.2 J/(g.°C) as the specific heat capacity of water and assume that
during this time the energy transferred between the contents of the cup and the surroundings
is negligible and that there is no heat loss to the cup.
(a) How much heat in joules is gained by the water?
(b) How much heat in joules is lost by the metal?
(c) What is the heat capacity of this piece of metal?
(d) If the mass of the metal is 196 grams, what is its specific heat capacity?
(e) What is the change of water's entropy? Note the volume of water stays the same.
(f) What is the change of total entropy of the system, including both water and metal?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Automotive Technology: A Systems Approach (MindTa…
Mechanical Engineering
ISBN:
9781133612315
Author:
Jack Erjavec, Rob Thompson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Automotive Technology: A Systems Approach (MindTa…
Mechanical Engineering
ISBN:
9781133612315
Author:
Jack Erjavec, Rob Thompson
Publisher:
Cengage Learning