2. The following reactions have been observed to be spontaneous: For each reaction, decide which OA is stronger and which RA is stronger. An example is shown. A + B* - A* + B OA: RA: C* + A* - C + A OA: RA: D+ +B + D + B+ OA: RA:
2. The following reactions have been observed to be spontaneous: For each reaction, decide which OA is stronger and which RA is stronger. An example is shown. A + B* - A* + B OA: RA: C* + A* - C + A OA: RA: D+ +B + D + B+ OA: RA:
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter18: Principles Of Chemical Reactivity: Entropy And Free Energy
Section18.7: Calculating And Using Standard Free Energies, Δfg˚
Problem 2RC
Related questions
Question
The following reactions (in the left column) have been observed to be spontaneous. For each reaction decide which OA is stronger and which RA is stronger in the right column. An example is shown.
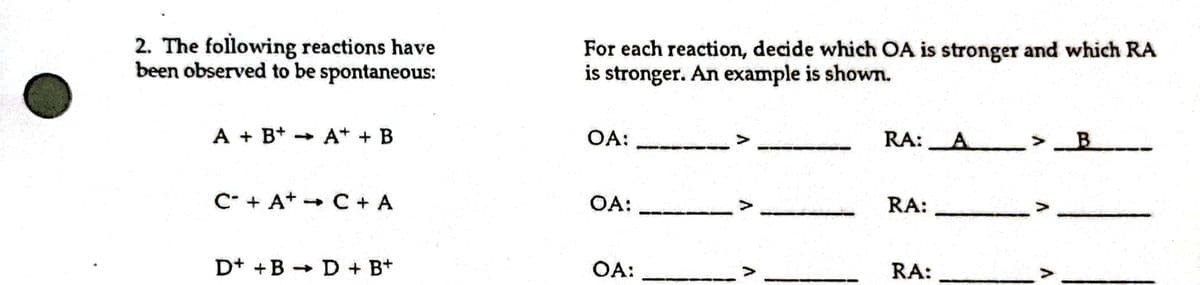
Transcribed Image Text:2. The following reactions have
been observed to be spontaneous:
For each reaction, decide which OA is stronger and which RA
is stronger. An example is shown.
A + B+ + A* + B
OA:
RA:A >
C* + A+ - C + A
OA:
RA:
D+ +B + D + B+
OA:
RA:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning