2. The reaction shown below was performed as part of a research program funded by the National Institute of Health (NIH) to develop therapeutic agents for the treatment of cocaine addiction. ● ● H ● H- H 1₂ H HO O H O NaHCO3 1 sodium bicarbonate = mild base Using what you have learned in Chapter 8 about the addition reactions of halogens with alkenes draw a complete arrow-pushing mechanism for this process to include the following: FO obranysl Show all arrows for each mechanistic step, to illustrate correct electron flow Structures of all reaction intermediates formed in the mechanism must be shown correctly, including the key cyclic iodonium ion intermediate Underline the iodonium ion intermediate that is formed in the reaction Stereochemistry of reaction intermediates consistent with the product stereochemistry shown Formal charges where applicable
2. The reaction shown below was performed as part of a research program funded by the National Institute of Health (NIH) to develop therapeutic agents for the treatment of cocaine addiction. ● ● H ● H- H 1₂ H HO O H O NaHCO3 1 sodium bicarbonate = mild base Using what you have learned in Chapter 8 about the addition reactions of halogens with alkenes draw a complete arrow-pushing mechanism for this process to include the following: FO obranysl Show all arrows for each mechanistic step, to illustrate correct electron flow Structures of all reaction intermediates formed in the mechanism must be shown correctly, including the key cyclic iodonium ion intermediate Underline the iodonium ion intermediate that is formed in the reaction Stereochemistry of reaction intermediates consistent with the product stereochemistry shown Formal charges where applicable
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter16: Aldehydes And Ketones
Section: Chapter Questions
Problem 16.65P: All rearrangements we have discussed so far have involved generation of an electron-deficient carbon...
Related questions
Question
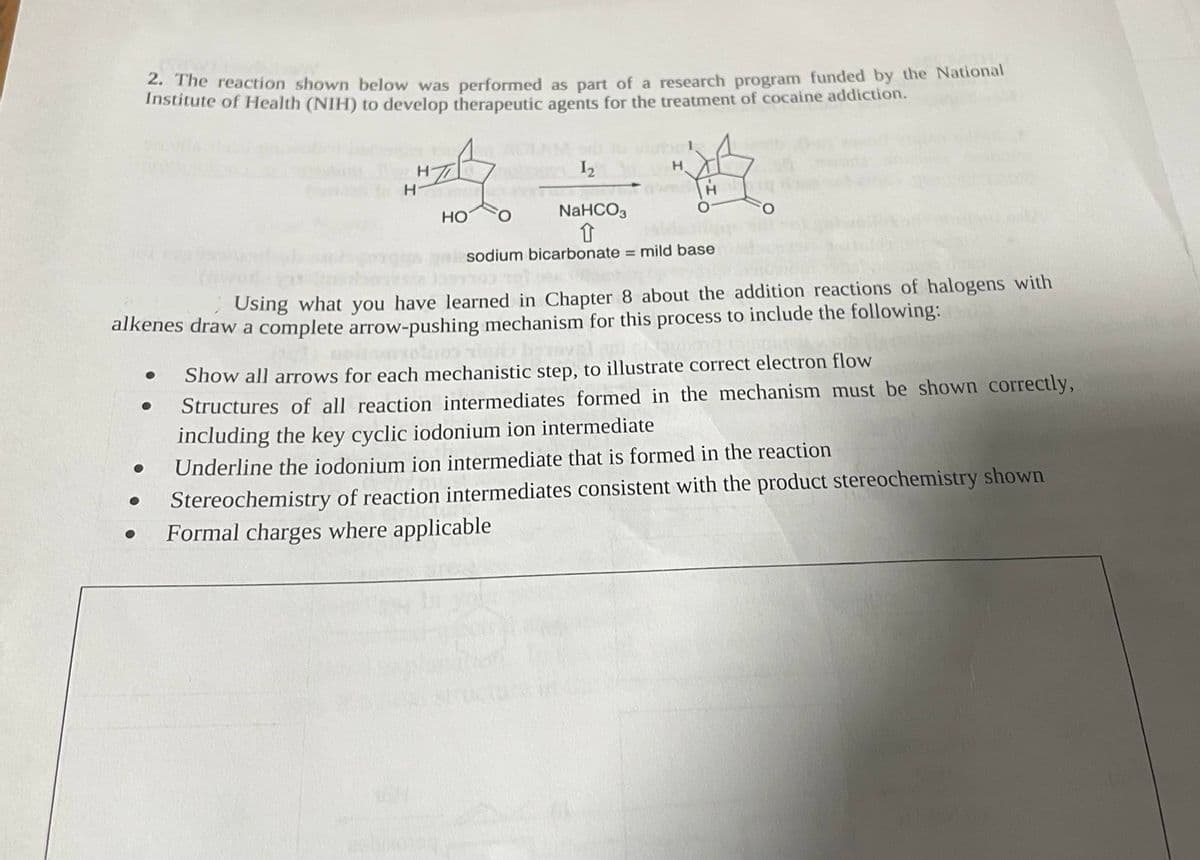
Transcribed Image Text:2. The reaction shown below was performed as part of a research program funded by the National
Institute of Health (NIH) to develop therapeutic agents for the treatment of cocaine addiction.
●
HT
●
H
1₂
NaHCO3
↑
sodium bicarbonate = mild base
H
AFH
O
HO O
Using what you have learned in Chapter 8 about the addition reactions of halogens with
alkenes draw a complete arrow-pushing mechanism for this process to include the following:
Show all arrows for each mechanistic step, to illustrate correct electron flow
Structures of all reaction intermediates formed in the mechanism must be shown correctly,
including the key cyclic iodonium ion intermediate
Underline the iodonium ion intermediate that is formed in the reaction
Stereochemistry of reaction intermediates consistent with the product stereochemistry shown
Formal charges where applicable
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning