2. The titration of 100 mL of carbonic acid with 1.0 M NaOH is shown below. 0.10 L Carbonic a. Label the equivalence point on the graph and circle the buffering region b. Based on the given information what is the pka1 of carbonic acid? (Explain/Show your work) c. Determine the initial concentration of the carbonic acid (Show your work) E 9.0 8.0 7.0 6.0 5.0 4.0 0.0 2.5 5.0 NaOH (mL) 7.5 10.0
2. The titration of 100 mL of carbonic acid with 1.0 M NaOH is shown below. 0.10 L Carbonic a. Label the equivalence point on the graph and circle the buffering region b. Based on the given information what is the pka1 of carbonic acid? (Explain/Show your work) c. Determine the initial concentration of the carbonic acid (Show your work) E 9.0 8.0 7.0 6.0 5.0 4.0 0.0 2.5 5.0 NaOH (mL) 7.5 10.0
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter43: Analysis For Vitamin C
Section: Chapter Questions
Problem 3ASA
Related questions
Question
question 2 a-d
Transcribed Image Text:1:38 PM Tue Aug 16
X
Chem 348
1review
PDF - 91 KB
Review of Concepts
1. To make beer and bread, yeast convert acetaldehyde to ethanol.
a. Is the acetaldehyde being oxidized or reduced?
b. How many electrons are being transferred?
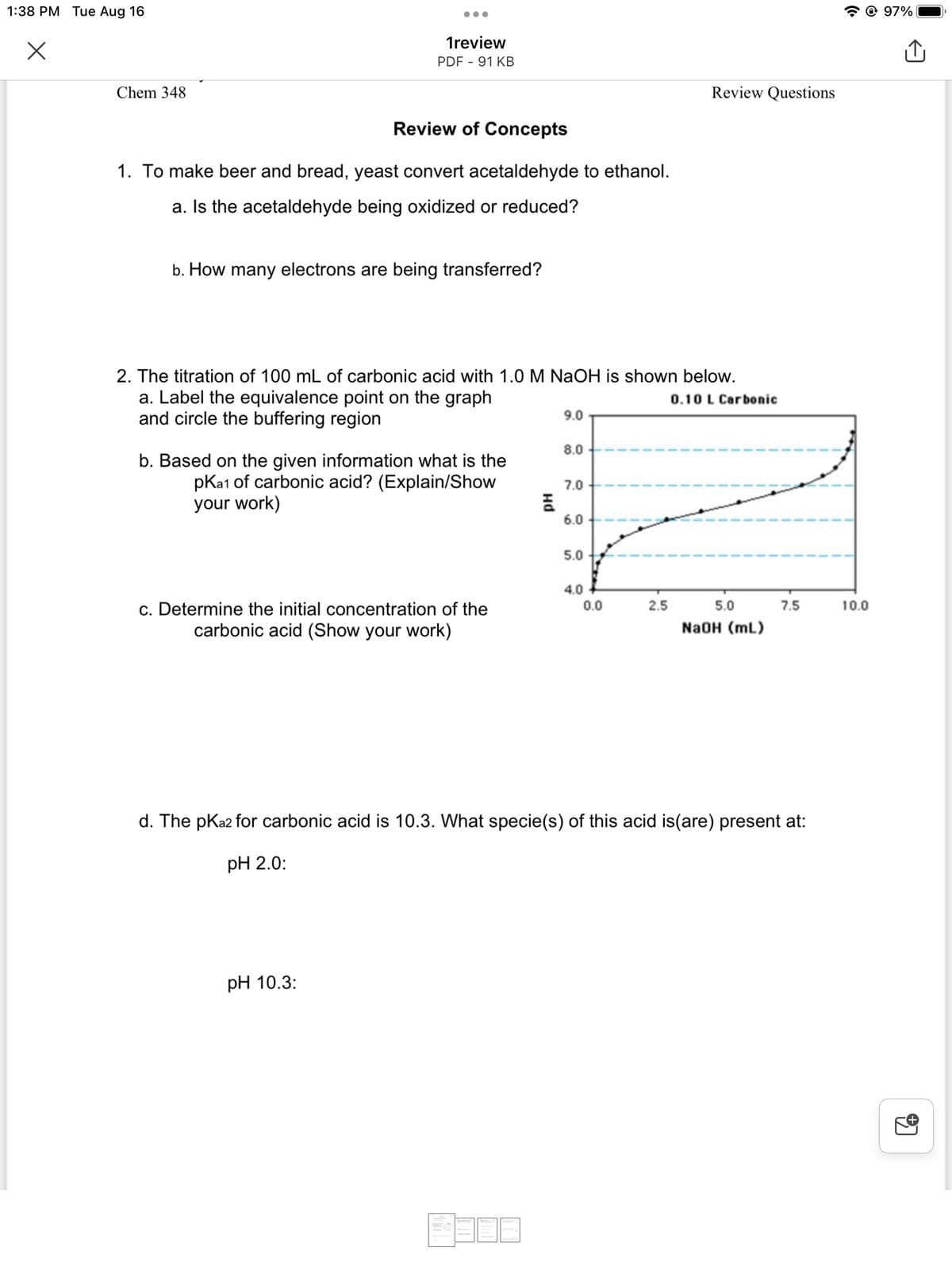
2. The titration of 100 mL of carbonic acid with 1.0 M NaOH is shown below.
a. Label the equivalence point on the graph
and circle the buffering region
0.10 L Carbonic
b. Based on the given information what is the
pka1 of carbonic acid? (Explain/Show
your work)
c. Determine the initial concentration of the
carbonic acid (Show your work)
pH 10.3:
Hd
9.0
8.0
7.0
6.0
5.0
4.0
0.0
Review Questions
2.5
5.0
NaOH (mL)
7.5
d. The pka2 for carbonic acid is 10.3. What specie(s) of this acid is(are) present at:
pH 2.0:
✪ 97%
10.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole