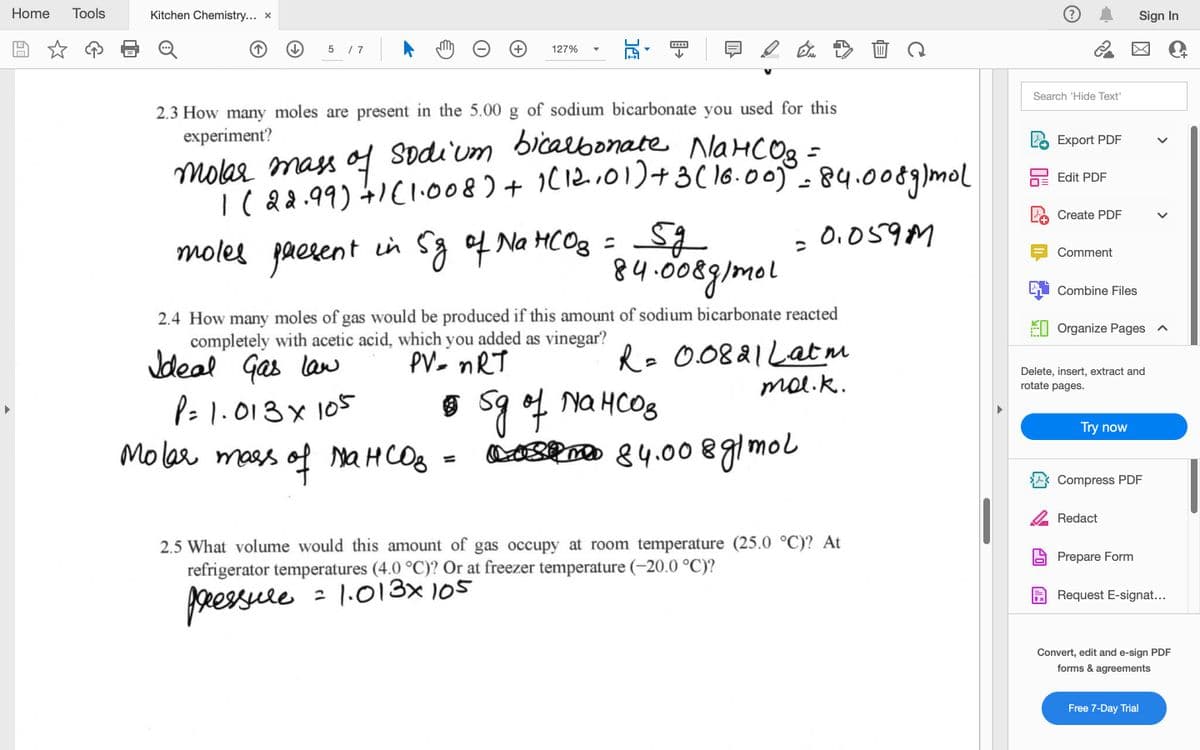
2.4 How many moles of gas would be produced if this amount of sodium bicarbonate reacted completely with acetic acid, which you added as vinegar? Jdeal Gas law Re 0.0821Lat m mol.k. PV- nRT P= 1.013x 105 Molar mass of MaHCOB Na HCO8 OBRD 84.00 glmol %3D 2.5 What volume would this amount of gas occupy at room temperature (25.0 °C)? At refrigerator temperatures (4.0 °C)? Or at freezer temperature (-20.0 °C)? 2 1.013x 105 ppessre
2.4 How many moles of gas would be produced if this amount of sodium bicarbonate reacted completely with acetic acid, which you added as vinegar? Jdeal Gas law Re 0.0821Lat m mol.k. PV- nRT P= 1.013x 105 Molar mass of MaHCOB Na HCO8 OBRD 84.00 glmol %3D 2.5 What volume would this amount of gas occupy at room temperature (25.0 °C)? At refrigerator temperatures (4.0 °C)? Or at freezer temperature (-20.0 °C)? 2 1.013x 105 ppessre
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter20: Environmental Chemistry-earth's Environment, Energy, And Sustainability
Section: Chapter Questions
Problem 41PS
Related questions
Question
100%
Hi Can you please help me with question 2.4 and 2.5 .
Thanks
Transcribed Image Text:Home
Tools
Kitchen Chemistry... x
Sign In
/ 7
127%
Search 'Hide Text'
2.3 How many moles are present in the 5.00 g of sodium bicarbonate you used for this
experiment?
LO Export PDF
sodium bicalbonate NaHCO.
molar mass o
|(82.99) 4IC1.008) + 1(12..01)+3( 16.00. 84.0089)mol
l
Edit PDF
Create PDF
moles paesent in Sg of Na HCOg = S
84.008g/mel
0.059M
Comment
Combine Files
2.4 How many moles of gas would be produced if this amount of sodium bicarbonate reacted
completely with acetic acid, which you added as vinegar?
EI Organize Pages ^
Ro 0.0821Latm
mal.k.
PV- nRT
Jdeal Gas law
P=1.013x 105
Delete, insert, extract and
rotate pages.
o s9 f Na HCOS
Poo 84.008gimol
Try now
Molar mass of MAHCOB =
Compress PDF
2 Redact
2.5 What volume would this amount of gas occupy at room temperature (25.0 °C)? At
refrigerator temperatures (4.0 °C)? Or at freezer temperature (-20.00 °C)?
2 1.013x 105
Prepare Form
pressure
Request E-signat...
Convert, edit and e-sign PDF
forms & agreements
Free 7-Day Trial
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning