2.5.1 Boron has two naturally occurring isotopes, 1°B and "B, which have masses 10.0129 and 11.0093 amu, respec- tively. Given the average atomic mass of boron (10.81 amu), determine the percent abundance of each isotope. a) 50% 1ºB, 50% "B d) 93% 'ºB, 7% B b) 20% 1°B, 80% "B e) 22% 1°в, 78% "в с) 98% 1°в, 2% "в
2.5.1 Boron has two naturally occurring isotopes, 1°B and "B, which have masses 10.0129 and 11.0093 amu, respec- tively. Given the average atomic mass of boron (10.81 amu), determine the percent abundance of each isotope. a) 50% 1ºB, 50% "B d) 93% 'ºB, 7% B b) 20% 1°B, 80% "B e) 22% 1°в, 78% "в с) 98% 1°в, 2% "в
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter2: Atoms
Section: Chapter Questions
Problem 2.83P: 2-83 The natural abundance of boron isotopes is as follows: 19.9sf boron-l0 (10.013 amu) and 80.1%...
Related questions
Question
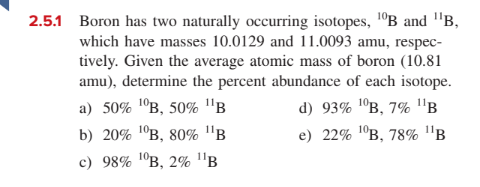
Transcribed Image Text:2.5.1 Boron has two naturally occurring isotopes, 1°B and "B,
which have masses 10.0129 and 11.0093 amu, respec-
tively. Given the average atomic mass of boron (10.81
amu), determine the percent abundance of each isotope.
a) 50% 1ºB, 50% "B
d) 93% 'ºB, 7% B
b) 20% 1°B, 80% "B
e) 22% 1°в, 78% "в
с) 98% 1°в, 2% "в
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning