Isotopic abundance (3 decimal places) No. of No. of Atomic A. Element Isotope neutrons mass (u) protons 34.96885 75.5% 35 CI 37CI 63Cu 17CI 36.96590 24.5% 62.92960 69.15% 29 Cu 65Cu 64.92779 30.85% 78.91834 50.8% 7°Br 8Br 64Zn 35 Br 80.91629 49.2% 63.92914 49.17% 66Zn 65.92603 27.73% 66.92713 4.04% 30Zn 68Zn 67.92484 18.45% 70Zn 69.92532 0.61% 56FE 53.93961 5.845% 91.754% 54Fe 57FE 55.93494 26 Fe 56.93539 2.119% 58Fe 57.93327 0.282%
Isotopic abundance (3 decimal places) No. of No. of Atomic A. Element Isotope neutrons mass (u) protons 34.96885 75.5% 35 CI 37CI 63Cu 17CI 36.96590 24.5% 62.92960 69.15% 29 Cu 65Cu 64.92779 30.85% 78.91834 50.8% 7°Br 8Br 64Zn 35 Br 80.91629 49.2% 63.92914 49.17% 66Zn 65.92603 27.73% 66.92713 4.04% 30Zn 68Zn 67.92484 18.45% 70Zn 69.92532 0.61% 56FE 53.93961 5.845% 91.754% 54Fe 57FE 55.93494 26 Fe 56.93539 2.119% 58Fe 57.93327 0.282%
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.12QAP
Related questions
Question
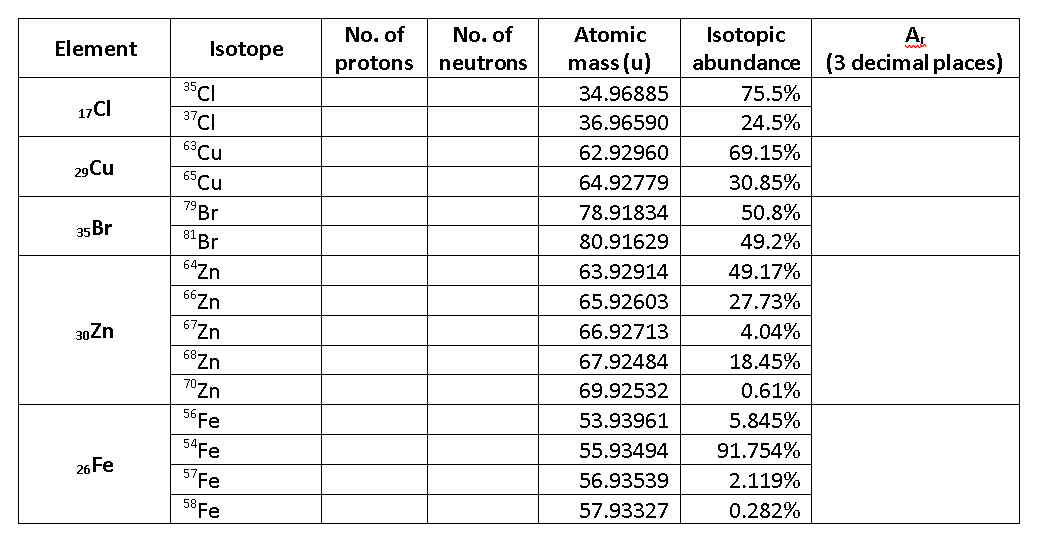
Complete the table.
|
Element |
Isotope |
No. of protons |
No. of neutrons |
|
Isotopic abundance |
Ar (3 decimal places) |
|
17Cl |
35Cl |
|
|
34.96885 |
75.5% |
|
|
37Cl |
|
|
36.96590 |
24.5% |
||
|
29Cu |
63Cu |
|
|
62.92960 |
69.15% |
|
|
65Cu |
|
|
64.92779 |
30.85% |
||
|
35Br |
79Br |
|
|
78.91834 |
50.8% |
|
|
81Br |
|
|
80.91629 |
49.2% |
||
|
30Zn |
64Zn |
|
|
63.92914 |
49.17% |
|
|
66Zn |
|
|
65.92603 |
27.73% |
||
|
67Zn |
|
|
66.92713 |
4.04% |
||
|
68Zn |
|
|
67.92484 |
18.45% |
||
|
70Zn |
|
|
69.92532 |
0.61% |
||
|
26Fe |
56Fe |
|
|
53.93961 |
5.845% |
|
|
54Fe |
|
|
55.93494 |
91.754% |
||
|
57Fe |
|
|
56.93539 |
2.119% |
||
|
58Fe |
|
|
57.93327 |
0.282% |
Transcribed Image Text:No. of
No. of
Atomic
Isotopic
A,
Element
Isotope
protons
mass (u)
abundance (3 decimal places)
neutrons
35CI
37CI
34.96885
75.5%
17CI
36.96590
24.5%
62.92960
69.15%
29 Cu
65Cu
64.92779
30.85%
79Br
78.91834
50.8%
35 Br
80.91629
49.2%
6AZN
49.17%
63.92914
65.92603
27.73%
30Zn
67Zn
66.92713
4.04%
67.92484
18.45%
70Zn
56FE
54Fe
57FE
69.92532
0.61%
53.93961
5.845%
55.93494
91.754%
26 Fe
56.93539
2.119%
57.93327
0.282%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

