20 21 23 24 :0 F1 2 26 27 2 F2 14/ Select all answers that show the atoms or ions that are isoelectronic with each other Se2-, Kr2+ S2-, Cl-1 MacBook Air : DD J F9 #3 80 F3 L A12+, Mg2+ 000 000 F4 S4 $ 4 C % 5 F5 T A 6 F6 X & 7 এব F7 * 8 00 DII F8 ( 9 - O 0 F10 I' F11 +
20 21 23 24 :0 F1 2 26 27 2 F2 14/ Select all answers that show the atoms or ions that are isoelectronic with each other Se2-, Kr2+ S2-, Cl-1 MacBook Air : DD J F9 #3 80 F3 L A12+, Mg2+ 000 000 F4 S4 $ 4 C % 5 F5 T A 6 F6 X & 7 এব F7 * 8 00 DII F8 ( 9 - O 0 F10 I' F11 +
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter2: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 6ALQ: Explain why a graph of ionization energy versus atomic number (across a row) is not linear. Where...
Related questions
Question
Transcribed Image Text:LO
کارخانه فرار دولت موكا
20
21
23 24
:0
F1
30
2
26 27
@
2
F2
W
S
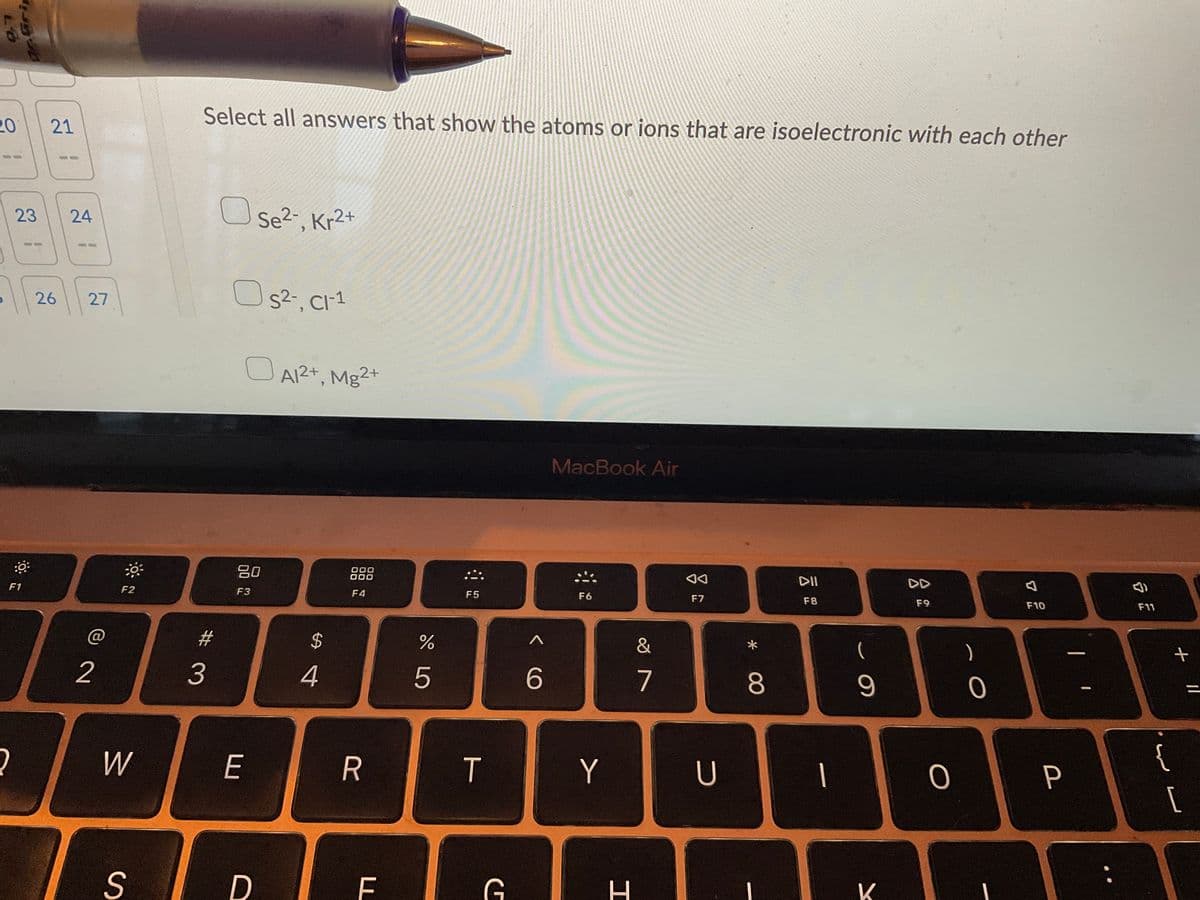
Select all answers that show the atoms or ions that are isoelectronic with each other
Se²-, Kr2+
S²-, Cl-1
OA1²+, Mg2+
MacBook Air
80
000
000
DD
F3
F4
F6
F9
3
E
D
$
4
R
F
do L
%
5
F5
T
G
< C
6
Y
H
&
7
AA
F7
U
*
8
DII
F8
1
9
K
O
O
I
7
F10
P
1
F11
{
+
لار
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning