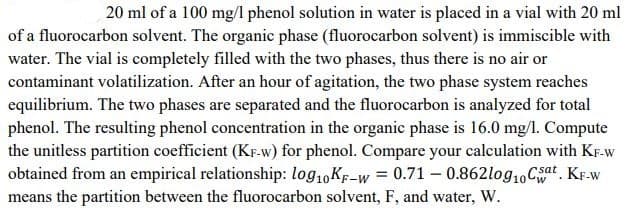
20 ml of a 100 mg/l phenol solution in water is placed in a vial with 20 ml of a fluorocarbon solvent. The organic phase (fluorocarbon solvent) is immiscible with water. The vial is completely filled with the two phases, thus there is no air or contaminant volatilization. After an hour of agitation, the two phase system reaches equilibrium. The two phases are separated and the fluorocarbon is analyzed for total phenol. The resulting phenol concentration in the organic phase is 16.0 mg/l. Compute the unitless partition coefficient (KF-W) for phenol. Compare your calculation with KF-W obtained from an empirical relationship: log10KF-W 0.71 - 0.862log10 CWsat. KF-W means the partition between the fluorocarbon solvent, F, and water, W. Only typed solution.
20 ml of a 100 mg/l phenol solution in water is placed in a vial with 20 ml of a fluorocarbon solvent. The organic phase (fluorocarbon solvent) is immiscible with water. The vial is completely filled with the two phases, thus there is no air or contaminant volatilization. After an hour of agitation, the two phase system reaches equilibrium. The two phases are separated and the fluorocarbon is analyzed for total phenol. The resulting phenol concentration in the organic phase is 16.0 mg/l. Compute the unitless partition coefficient (KF-W) for phenol. Compare your calculation with KF-W obtained from an empirical relationship: log10KF-W 0.71 - 0.862log10 CWsat. KF-W means the partition between the fluorocarbon solvent, F, and water, W.
Only typed solution.
Step by step
Solved in 3 steps with 9 images




